Detergent compositions containing thermobifida fusca lipase and methods of use thereof
A composition and detergent technology, applied in the direction of detergent composition, detergent compounding agent, chemical instrument and method, etc., can solve the problem of synthesizing complex toxic waste products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0184] The following examples are provided to demonstrate and illustrate certain preferred embodiments and aspects of the invention and should not be construed as limiting.
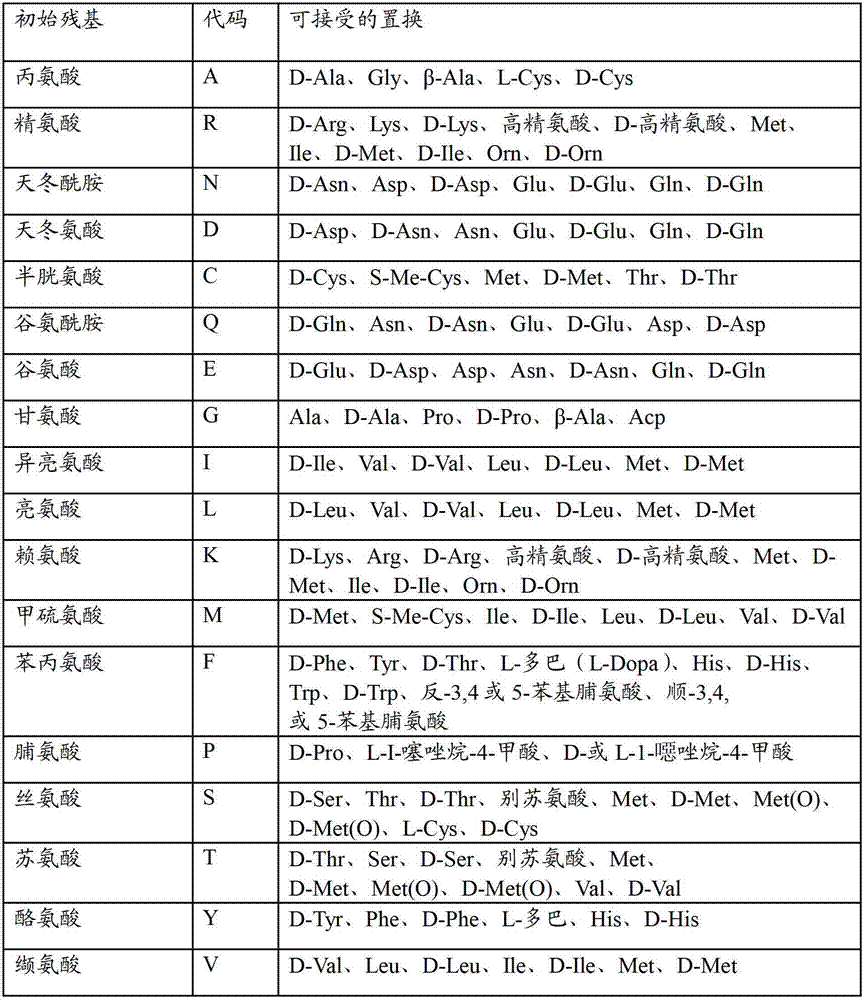
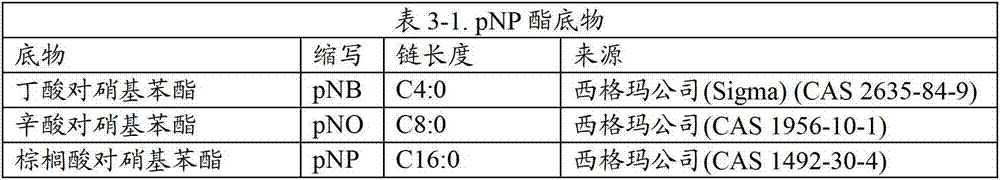
[0185]In the following experimental disclosures, the following abbreviations will be used: M (molar); mM (millimolar); μM (micromolar); nM (nanomolar); μmol (micromole); nmol (nanomole); gm (gram); mg (milligram); μg (microgram); pg (picogram); L (liter); ml and mL (milliliter); μl and μL (microgram); liter); cm (centimeter); mm (millimeter); μm (micrometer); nm (nanometer); U (unit); MW (molecular weight); sec (second); min(s) (minute); h(s) and hr(s) (hours); °C (degrees Celsius); QS (sufficient); ND (not performed); rpm (revolutions per minute); H 2 O (water); dH 2 O (deionized water); HCl (hydrochloric acid); aa (amino acid); bp (base pair); kb (kilobase pair); kD (kilodalton); MgCl 2 (magnesium chloride); NaCl (sodium chloride); w / v (weight / volume ratio); v / v (volume ratio); g (gravity); OD (optic...
example 1
[0187] Cloning and Expression of Lipase 2 (TfuLip2) from Thermobista spp.
[0188] The Thermobista spp. lipase 2 (or BTA-hydrolase 2) gene has been previously identified (Lykidis et al., J. Bacteriol, 189:2477-2486, 2007) , whose sequence is shown in GENBANK accession number YP_288944. The Bacillus subtilis expression vector p2JM103BBI (Vogtentanz et al., Prot. Expr. Purif., 55:40-52, 2007) was digested with restriction enzymes BssHII and HindIII. A DNA fragment lacking the sequence of the BCE103-BBI fusion gene was isolated and used as an expression backbone. This fragment was ligated to a synthetic gene encoding the TfuLip2 enzyme, resulting in a fusion of the N-terminus of the TfuLip2 polypeptide to the third amino acid of the B. subtilis AprE propeptide encoded by p2JM103BBI. The recombinant TfuLip2 protein produced in this way has three additional amino acids (Ala-Gly-Lys) at its amino terminus after cleavage by the native signal peptidase in the host.
[0189] The n...
example 2
[0197] Isolation and characterization of TfuLip2
[0198] Ultrafiltration concentrates were obtained from batch fermentation of B. subtilis expression strains on a 14 L scale. The clarified broth was used to characterize the recombinant TfuLip2 polypeptide.
[0199] To purify TfuLip2, the ultrafiltration concentrate was obtained from batch fermentation on a 14 L scale and diluted 5-fold with 50 mM Tris-HCl (pH 8.0) buffer, ammonium sulfate was added to a final concentration of 1 M. The precipitated pellet from ammonium sulfate precipitation was collected and used for further purification. A FastFlow Phenyl Sepharose column equilibrated with 1 M ammonium sulfate in 50 mM Tris-HCl (pH 8.0) buffer was used. Samples were loaded at half the equilibrium flow rate (12ml / min) and washed with equilibration buffer after loading. A gradient was used to decrease the concentration of ammonium sulfate in the buffer from 1M to 0M. Contaminating proteins in the column were washed with 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com