Cell inactivated vaccine, egg yolk antibody injection, and preparation method of cell inactivated vaccine
A technology of inactivated vaccine and yolk antibody, which is applied in the field of cell inactivated vaccine, yolk antibody injection and preparation thereof, can solve problems such as labor-intensive and time-consuming, and achieve the effect of reducing production steps, achieving remarkable curative effect, and reducing the probability of morbidity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
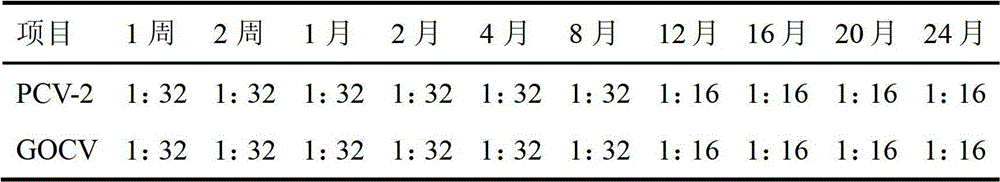
[0025] Example 1: Preparation of porcine circovirus cell inactivated vaccine in this example. The disease material was collected from pig farms with severe porcine circovirus disease in Shandong area. After clearing and filtering through a 0.22 μm filter membrane, a sensitive monolayer of PK-15 cells was inoculated. After the PK-15 cells were continuously passaged for 6 generations, part of the cells were frozen and thawed to extract the DNA of the PCV-2 virus, and the target gene was amplified with PCR primers specific to the PCV-2 virus ORF1 gene. The amplified product was cloned into the PMD-18T vector for sequencing, identified and named SD strain. Multiple viral epitopes with antigenic immune activity were screened out in this strain, and five antigenic epitopes were finally selected after specific primer PCR, product electrophoresis, sequencing, cloning, purification, and detection of protein immune activity of each epitope. site, each antigen site is connected by splic...
Embodiment 2
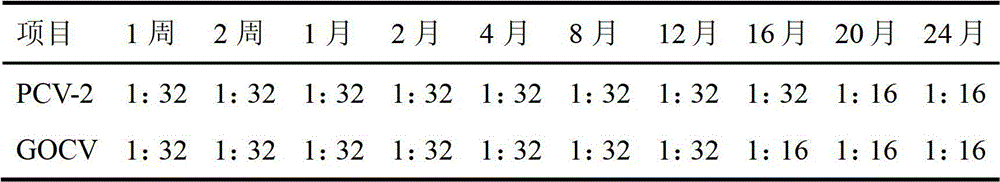
[0047] Embodiment 2: The preparation of porcine circovirus cell inactivated vaccine of the present embodiment collects disease material from pig farm that serious porcine circovirus takes place in Shandong area, and disease material is after homogenization, freeze-thaw repeatedly 3 times, high-speed centrifugation, take After the supernatant was filtered through a 0.22 μm filter membrane, a sensitive monolayer of PK-15 cells was inoculated. After the PK-15 cells were continuously passaged for 6 generations, part of the cells were frozen and thawed to extract the DNA of the PCV-2 virus, and the target gene was amplified with PCR primers specific to the PCV-2 virus ORF1 gene. The amplified product was cloned into the PMD-18T vector for sequencing, identified and named SD strain. Multiple viral epitopes with antigenic immune activity were screened out in this strain, and five antigenic epitopes were finally selected after specific primer PCR, product electrophoresis, sequencing, ...
Embodiment 3
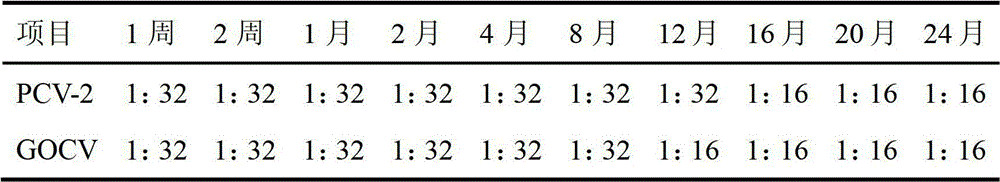
[0069] Embodiment 3: The preparation of porcine circovirus cell inactivated vaccine of this embodiment collects disease material from pig farm that serious porcine circovirus takes place in Shandong area, and disease material is after homogenization, freeze-thaw repeatedly 3 times, high-speed centrifugation, take After the supernatant was filtered through a 0.22 μm filter membrane, a sensitive monolayer of PK-15 cells was inoculated. After the PK-15 cells were continuously passaged for 6 generations, part of the cells were frozen and thawed to extract the DNA of the PCV-2 virus, and the target gene was amplified with PCR primers specific to the PCV-2 virus ORF1 gene. The amplified product was cloned into the PMD-18T vector for sequencing, identified and named SD strain. Multiple viral epitopes with antigenic immune activity were screened out in this strain, and five antigenic epitopes were finally selected after specific primer PCR, product electrophoresis, sequencing, cloning...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com