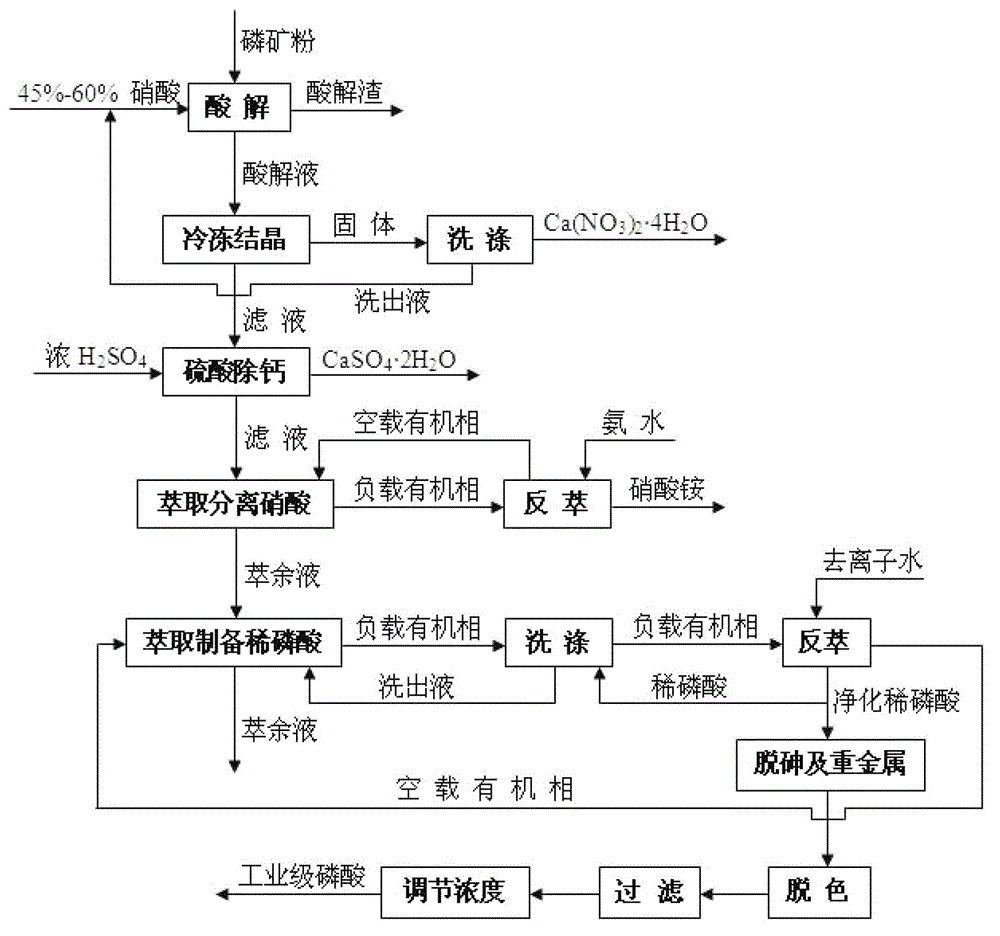
Method for preparing industrial-grade phosphoric acid by decomposing mid-low-grade phosphorite with nitric acid
A low-grade, industrial-grade technology, applied in the direction of phosphoric acid, phosphorus oxyacid, etc., can solve the problems of undisclosed purification treatment, high phosphoric acid extraction rate, unfavorable phosphoric acid yield, etc., to avoid the generation of phosphogypsum and improve economic benefits , highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] In this embodiment, the process steps of the method for preparing industrial-grade phosphoric acid by decomposing medium and low-grade phosphate rocks with nitric acid are as follows:
[0047] (1) Decompose phosphate rock with nitric acid
[0048] The phosphate rock powder is acidolyzed with nitric acid with a mass concentration of 45%, and the amount of nitric acid is: CaO, MgO, Fe in the phosphate rock powder 2 o 3 and Al 2 o 3 110% of the theoretical amount of nitric acid required for complete reaction with nitric acid; first, add the nitric acid into the acidolysis tank, then add phosphate rock powder to the nitric acid under stirring, and the acid hydrolysis reaction is carried out at normal pressure and 40°C under stirring Carry out, the time is 30min, after the reaction time expires, carry out vacuum filtration, the filtrate is the acid hydrolysis solution, and its contained substances (mass percentage, %) are as shown in Table 2:
[0049] Table 2 Compositio...
Embodiment 2
[0074] In this embodiment, the process steps of the method for preparing industrial-grade phosphoric acid by decomposing medium and low-grade phosphate rocks with nitric acid are as follows:
[0075] (1) Decompose phosphate rock with nitric acid
[0076] The phosphate rock powder is acidolyzed with nitric acid with a mass concentration of 55%, and the amount of nitric acid is: CaO, MgO, Fe in the phosphate rock powder 2 o 3 and Al 2 o 3 95% of the theoretical amount of nitric acid required for complete reaction with nitric acid; first add the nitric acid into the acidolysis tank, then add phosphate rock powder to the nitric acid under stirring, and the acid hydrolysis reaction is carried out at normal pressure and 50°C under stirring Carry out, the time is 60min, after the reaction time expires, carry out vacuum filtration, the filtrate is the acid hydrolysis solution, and its contained substances (mass percentage, %) are as shown in Table 7:
[0077] Table 7 Composition ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com