Preparation method for R-2-(4- hydroxyphenoxy) propionate
A technology of hydroxyphenoxy and R-2-, which is applied in the field of preparation of R-2-(4-hydroxyphenoxy) propionate, can solve the problem of low optical purity of the product, and reduce production cost, Small environmental pollution and strong splitting specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Slant medium: NaNO 3 2g, K 2 HPO 4 1g, KCl 5g, FeSO 4 0.01g, MgSO 4 0.5g, 30g sucrose, 15g agar, constant volume to 1L, natural pH, sterilized at 121°C for 20 minutes, cooled to make a slope after sterilization, inoculated with Aspergillus oryzae WZ007 (CCTCC No: M206105), cultured at 30°C for 3 days, as Slope activated seeds;
[0025] Liquid fermentation medium: glucose 20g, peptone 20g, sucrose 10g, urea 3g, NaCl 5g, k 2 HPO 4 2g, MgSO 4 1g,, (NH 4 ) 2 SO 4 5g, dilute to 1L, pH natural. The filling volume is 80ml of 500ml triangular flask, sterilized at 121°C for 20 minutes, cooled after sterilization, inoculated with slant seeds, cultivated at 30°C for 48 hours, filtered to obtain mycelium, and dried to obtain 4.2g of enzyme powder.
Embodiment 2
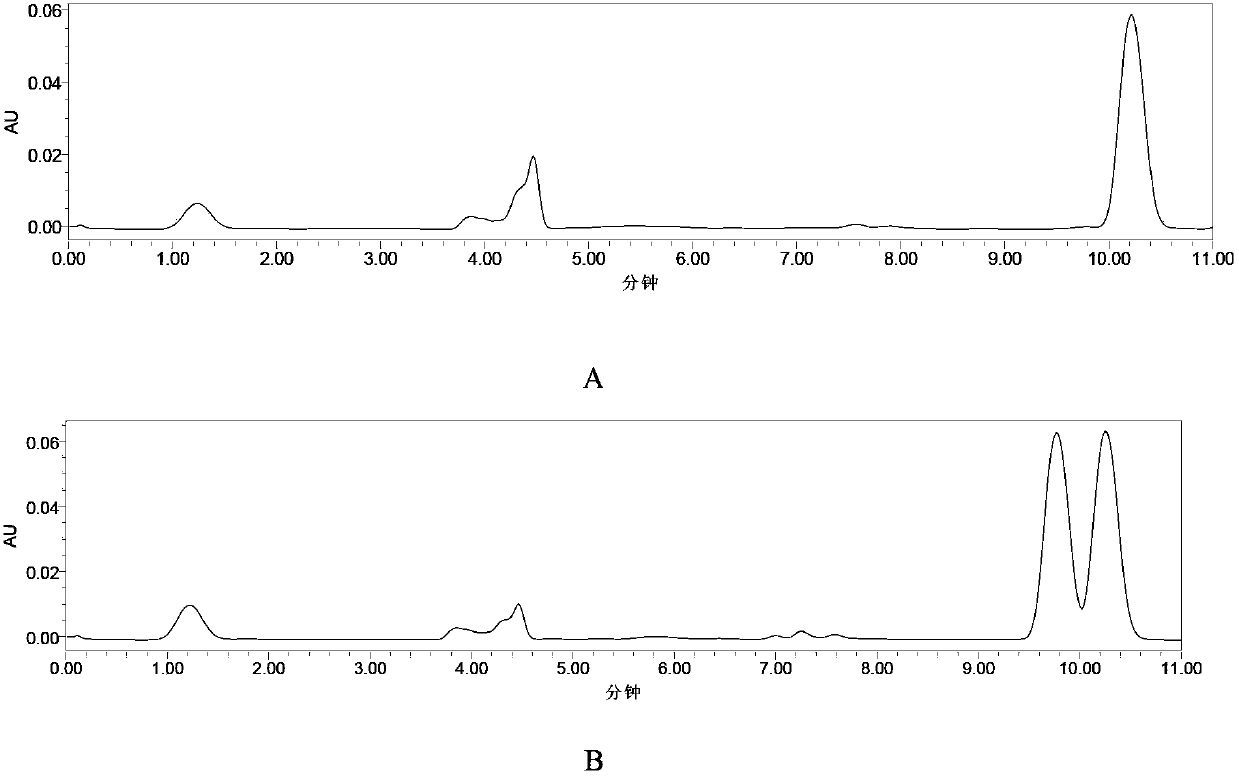
[0027] 0.1 g of Aspergillus oryzae WZ007 enzyme powder prepared by the method in Example 1 was added to 10 ml of phosphate buffer solution containing 20 g / L racemic 2-(4-hydroxyphenoxy) ethyl propionate, and added dropwise during the reaction The 1mol / L sodium hydroxide solution was maintained at about 7.0, and at 30°C, after 2 hours of reaction, the reaction conversion rate was 53.2%. After the reaction was completed, filter and remove the bacterial cells, add an equal amount of ethyl acetate to extract twice, then combine the extracts, and distill under reduced pressure at 0.1 MPa to obtain R-2-(4-hydroxyphenoxy) ethyl propionate. The enantiomeric excess value of R-2-(4-hydroxyphenoxy)propionate is 98.2%, and the yield is 90.2%. The liquid phase of ethyl 2-(4-hydroxyphenoxy)propionate before and after the reaction process For chromatogram see figure 1 .
Embodiment 3
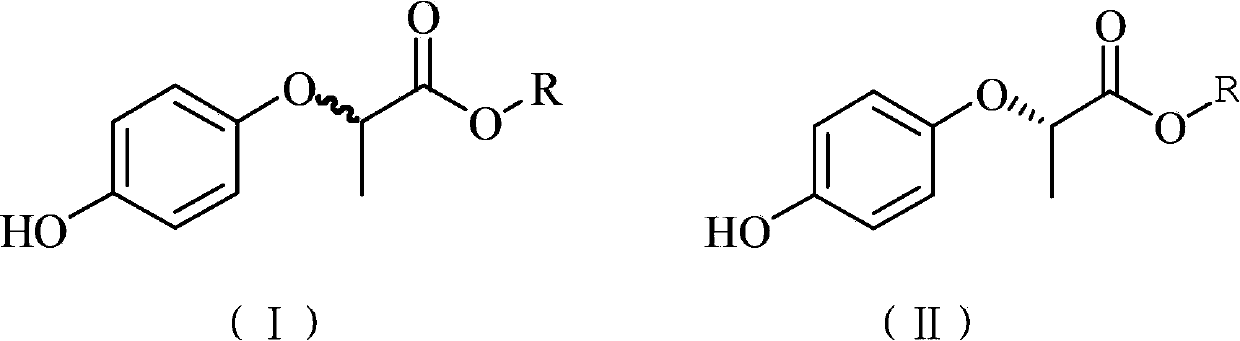
[0029] According to the method of Example 2, racemic 2-(4-hydroxyphenoxy) propionate with different R group structures was added as the reaction substrate, and other conditions remained unchanged. The reaction conversion and substrate enantiomeric excess values of this reaction are shown in Table 1. The results in Table 1 show that, in terms of reaction conversion rate and substrate enantiomeric excess value, the R group is isopropyl group is better than other groups.
[0030] Table 1: Enzyme conversion results of different substrate structures
[0031]
[0032] Example 3:
[0033] According to the method in Example 2, the transformed reaction solution was reacted, and the bacterial cells obtained by filtration were rinsed with a buffer solution and then added with a new reaction substrate to continue the reaction. Repeat this process and reuse it 5 times. The obtained enzymatic hydrolysis results are shown in Table 2. After using Aspergillus oryzae WZ007 enzyme powder...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com