HIV latency-resistant effective part of euphorbia and use thereof
An effective part, Euphorbia technology, applied in the field of medicine, can solve the problems of large toxic and side effects, unsatisfactory and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Preparation of Effective Fraction of Euphorbia for Anti-HIV Latency
[0076] Euphorbia (1kg, powder or chopped medicinal material) was extracted with 95% ethanol and refluxed (8L x 3), and the ethanol was recovered to obtain ethanol extract (0.26kg).
[0077] The ethanol extract was dissolved in 300 mL of hot water, and extracted with ethyl acetate (300 mL x 3) after cooling to obtain an ethyl acetate extract (47 g).
[0078] The ethyl acetate extract was subjected to silica gel column chromatography (2kg, column diameter: 10.0cm), eluted with petroleum ether: ethyl acetate (100:1) (20L) to remove the oily part, and then dichloromethane: methanol (1: 2) (20L) was eluted to obtain the effective fraction (43.4g).
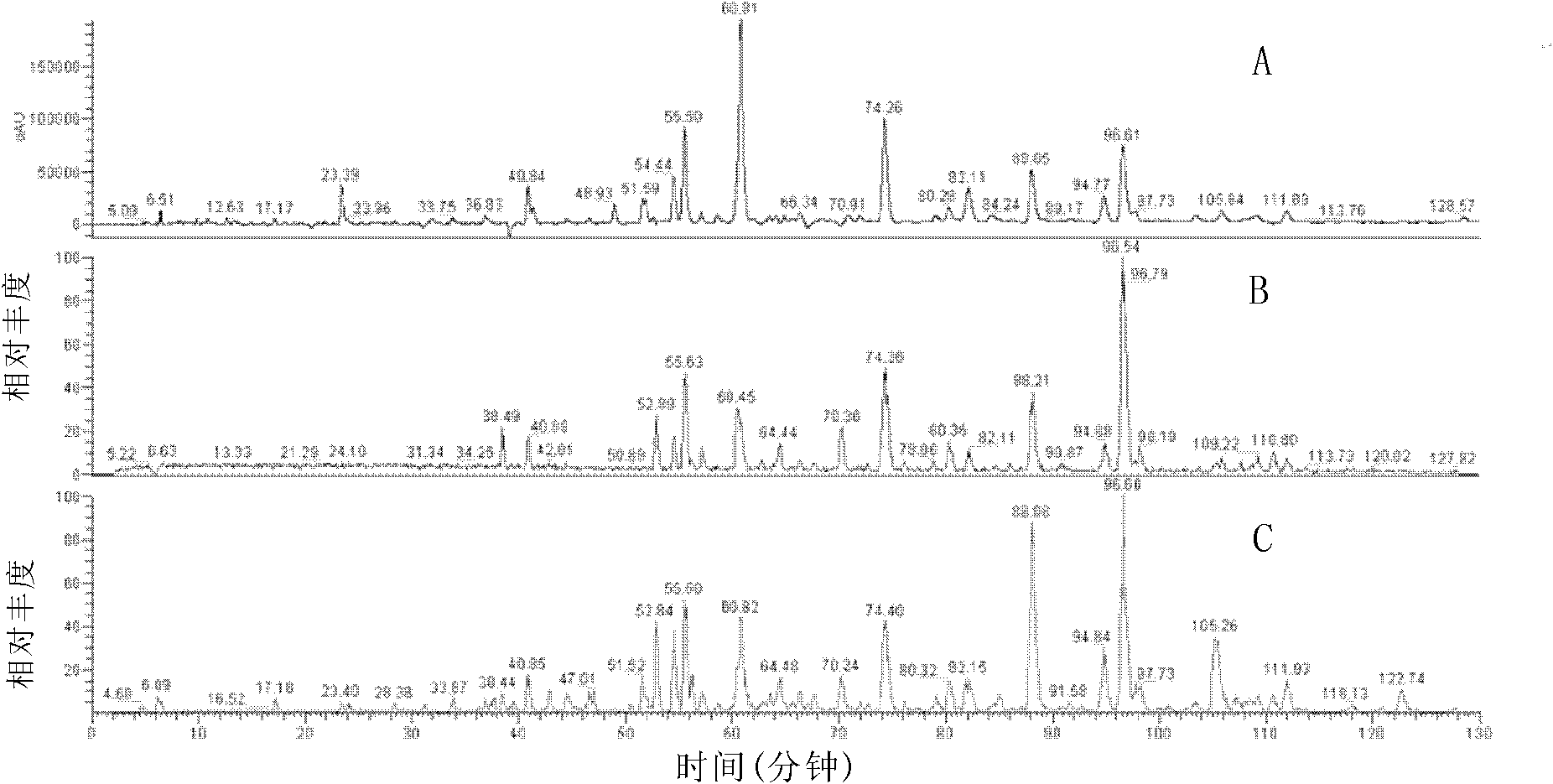
[0079] Carry out LC-MS to the obtained effective part, the method is as follows:
[0080] Analytical chromatographic conditions:
[0081] The chromatographic column was Zorbax SB-C18column (5 μm, 250mm×4.6mm; Agilent Technologies, USA); the mobile phase was ...
Embodiment 2
[0086] Preparation of effective fraction of Kansui anti-HIV latent effect
[0087] Gansui (1kg, powdered or chopped medicinal material) was extracted with water (10L x 2) and concentrated to obtain a water extract (0.33kg).
[0088] The water extract was dissolved in 300mL hot water (90°C), cooled and subjected to macroporous adsorption resin chromatography (1L), followed by 60% ethanol-water (4L) and 90% ethanol-water (4L) to obtain 90% ethanol-water The water eluate (49g) is the active part.
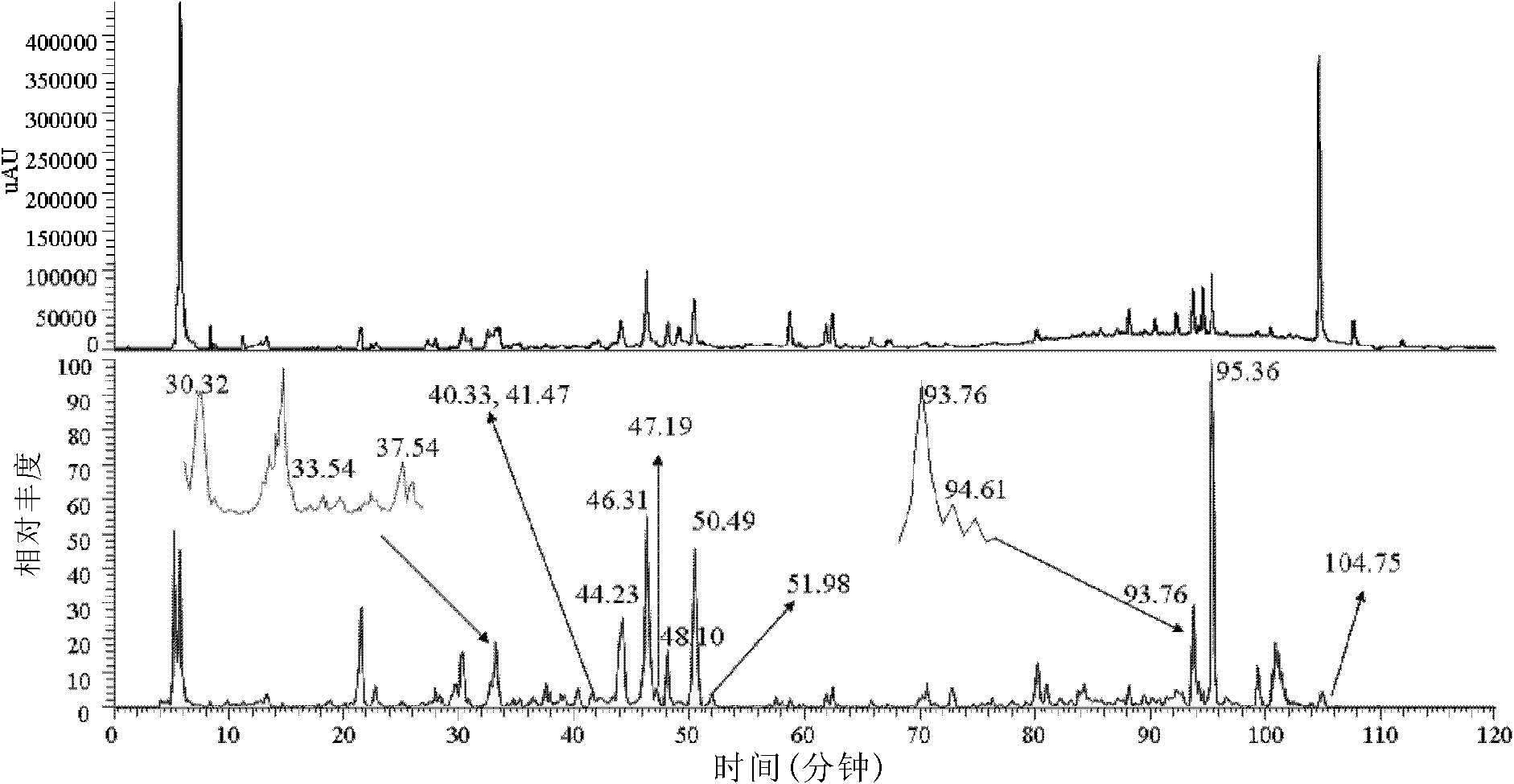
[0089] Carry out LC-MS to the obtained effective part, the method is as follows:
[0090] Analytical chromatographic conditions:
[0091] The chromatographic column was Zorbax SB-C18column (5 μm, 250mm×4.6mm; Agilent Technologies, USA); the mobile phase was A phase 0.5% acetic acid water, and B phase was 0.5% acetic acid acetonitrile. The gradient is as follows: from 25% Phase B to 40% Phase B in 30min, from 40% Phase B to 50% Phase B in 30min-45min, from 50% Phase B to 70% Phase B ...
Embodiment 3
[0096] Effects of effective fractions on HIV latency-induced activation
[0097] 3.1. Method
[0098] C11 cell is a latently infected cell model of HIV, which is obtained by infecting the human T lymphocyte cell line Jurkat with HIV-1 lentivirus carrying the EGFP reporter gene, through cell sorting and HIV integration detection, and has HIV integration but no expression EGFP T lymphocyte line. C11 cells are Jurkat stable strains that are infected by the HIV lentivirus carrying the reporter gene GFP and do not express at the same time, so they are used for screening and activating HIV-1 drugs for latent infection (see application number 200810038851.X, invention title "a screening for activating latent infection HIV-1 compound T lymphocytes and its preparation method" Chinese patent application), the preservation number is CCTCC NO.C200821.
[0099] In this example, 2×10 per well 4 C11 cells were planted in a 96-well plate, and 100 μl of 1640 medium (Gibco) containing 10% FB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com