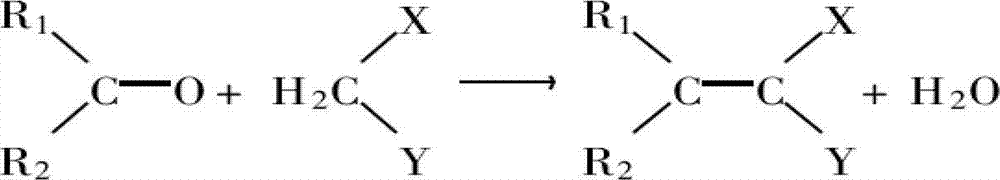
Organic solid base catalyst for synthesizing alpha-cyanoethyl cinnamate, and preparation method and application thereof
A technology of ethyl cinnamate and organic solids, which is applied in the chemical industry, can solve the problems of complicated separation, difficult separation, and harsh preparation process requirements, and achieve the effect of simple preparation method, easy operation, and good reusability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation and application of organic solid base catalyst
[0032] Add 10.0g of chloropropyltrimethoxysilane to 60.0g of cyclohexane to prepare a solution, and ultrasonically (KQ-50DB desktop CNC ultrasonic cleaner, working frequency 40KHz) disperses evenly, then add 10.0g of silica gel to it, and seal it. The reaction system was ultrasonically oscillated for 1 h. The sample was extracted with toluene for 8 hours, and dried in vacuum at 100°C for 8 hours. Get Cl / SiO 2 . Then 4.0g imidazole was added to 50g cyclohexane solution, and the above 10.0gCl / SiO 2 Dip it in, and seal it. After the reaction system was ultrasonically oscillated for 0.5h, 6g of n-butane bromide was added, and after ultrasonically oscillating for 1h, 2g of NaOH was added for ultrasonically oscillating for 0.5h, the sample was extracted with toluene for 8h, and vacuum-dried at 100°C for 8h. That is, the catalyst for this reaction is obtained. Add benzaldehyde, ethyl cyanoacetate ...
Embodiment 2
[0033] Embodiment 2: Preparation and application of organic solid base catalyst
[0034] Add 10.0g of chloropropyltrimethoxysilane to 65g of cyclohexane to prepare a solution, and ultrasonically (KQ-50DB desktop CNC ultrasonic cleaner, working frequency 40KHz) disperses evenly, then add 11.5g of silica gel to it, and seal it. The reaction system was ultrasonically oscillated for 1.2 h. The sample was extracted with toluene for 9 hours, and dried in vacuum at 95°C for 9 hours. Get Cl / SiO 2 . Then 5g imidazole was added to 50g cyclohexane solution, and the above 10.0gCl / SiO 2 Dip it in, and seal it. After the reaction system was ultrasonically oscillated for 0.6h, 7g of n-butane bromide was added. After ultrasonically oscillating for 1.1h, 3g of NaOH was added for ultrasonically oscillating for 0.6h. The sample was extracted with toluene for 9h and vacuum-dried at 95°C for 9h. That is, the reaction catalyst is obtained. Add methanol, propylene oxide and catalyst into a 100...
Embodiment 3
[0035] Embodiment 3: Preparation and application of organic solid base catalyst
[0036] Add 10.0g of chloropropyltrimethoxysilane to 70g of cyclohexane to prepare a solution, and ultrasonically (KQ-50DB desktop CNC ultrasonic cleaner, working frequency 40KHz) disperses evenly, then add 13.0g of silica gel to it, and seal it. The reaction system was ultrasonically oscillated for 1.4 h. The sample was extracted with toluene for 10 hours, and dried in vacuum at 90°C for 10 hours. Get Cl / SiO 2 . Then 6g of imidazole was added to 50g of cyclohexane solution, and the above 10.0g of Cl / SiO 2 Dip it in, and seal it. After the reaction system was ultrasonically oscillated for 0.8h, 8g of n-butane bromide was added. After ultrasonically oscillating for 1.2h, 4g of NaOH was added for ultrasonically oscillating for 0.7h. The sample was extracted with toluene for 10h, and vacuum-dried at 90°C for 10h. That is, the reaction catalyst is obtained. Add methanol, propylene oxide and cata...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com