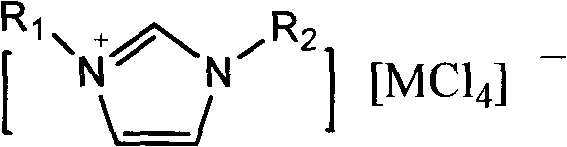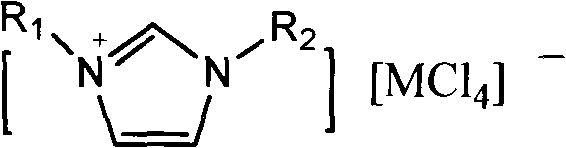New method for preparing p-chlorotoluene through selective chlorination of toluene
A p-chlorotoluene, selective technology, applied in the chemical field, can solve the problems of high cost, large amount of molecular sieve, etc., and achieve the effect of easy separation, small amount of catalyst, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 50g of toluene, 80mg of 1-methyl-3-n-hexylimidazolium tetrachloride iron salt, 1-methyl-3-n-hexylimidazolium chloride salt ([HeMIM]Cl) and evenly and slowly pass chlorine gas for 8 hours under dark stirring , the whole process temperature is controlled at 0°C, after the reaction is completed, filter, wash with water, dry with anhydrous sodium sulfate, and filter. Sampling was carried out for gas chromatography detection, and the toluene conversion rate was 95%, and the content ratio of p-chlorotoluene and o-chlorotoluene was 3.1:1.
Embodiment 2
[0022] Weigh 50g of toluene, 80mg of 1-methyl-3-n-octylimidazolium aluminum tetrachloride ionic liquid, 1-methyl-3-n-hexylimidazolium chloride salt ([HeMIM]Cl) and evenly and slowly pass chlorine gas under dark stirring For 8 hours, the temperature of the whole process was controlled at 25°C. After the reaction was completed, it was filtered, washed with water, dried over anhydrous sodium sulfate, and filtered. Sampling was carried out for gas chromatography detection, and the toluene conversion rate was 92%, and the content ratio of p-chlorotoluene and o-chlorotoluene was 2.6:1.
Embodiment 3
[0024] Weigh 50g of toluene, 80mg of 1-methyl-3-n-hexylimidazolium tetrachloride aluminum salt, 1-methyl-3-n-hexylimidazolium chloride salt ([HeMIM]Cl) and evenly and slowly pass chlorine gas for 8 hours under dark stirring , the temperature of the whole process is controlled at 50°C. After the reaction is completed, filter, wash with water, dry with anhydrous sodium sulfate, and filter. Sampling was carried out for gas chromatography detection, the toluene conversion rate was 96%, and the content ratio of p-chlorotoluene and o-chlorotoluene was 2.8:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com