Preparation method of doripenem
A C1-C6, allyl technology, applied in the preparation field of carbapenem compound doripenem, can solve the problems of complicated operation, fast reaction speed, difficult to remove, etc. The effect of product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of doripenem
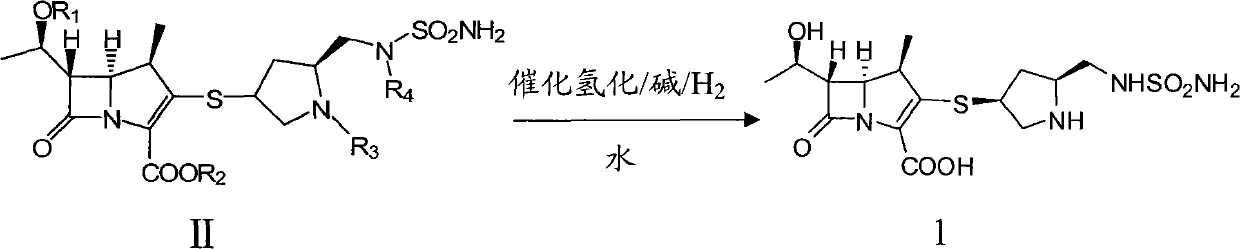
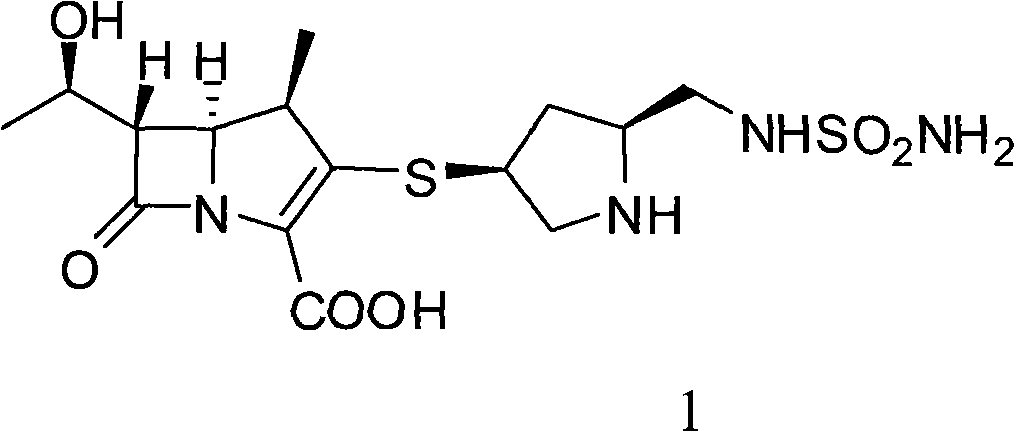
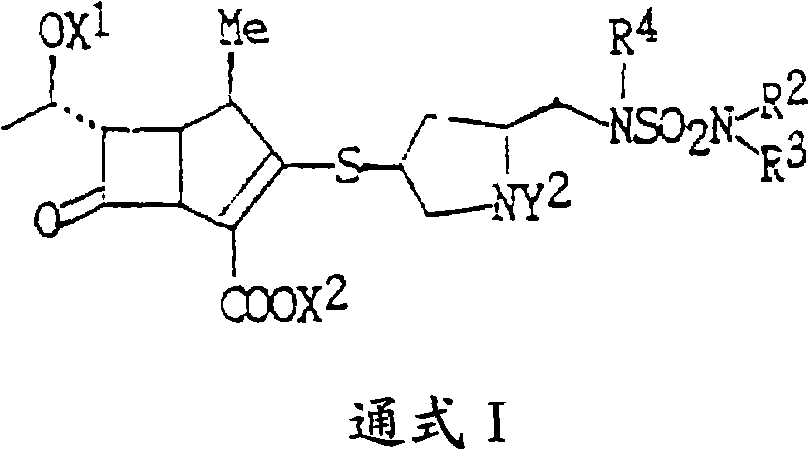
[0042] Add 1.0kg (1.36mol) of doripenem intermediate 2, 10% Pd / CO 0.5Kg, 0.32L (2.75mol) of 2,6-lutidine, 15L of deionized water, and nitrogen into a 50L hydrogenation kettle. Substitute several times, replace with hydrogen several times, add hydrogen to the pressure of 2.0Mpa in the kettle, react at 25°C for 3h, filter the reaction solution, recover and reuse palladium carbon, add 45L isopropanol to the filtrate, stir and analyze at 0-5°C After crystallization for 5 hours, 0.42Kg of doripenem was suction filtered, the molar yield was 73.5%, the HPLC purity was 99.2%, and the heavy metal content was <10ppm.
Embodiment 2
[0043] Embodiment 2: Preparation of doripenem
[0044] Add 1.0kg (1.36mol) of doripenem intermediate 2, 10% Pd / CO 0.5Kg, 0.08L (0.68mol) of 2,6-lutidine, 15L of deionized water, and nitrogen into a 50L hydrogenation kettle. Substitute several times, replace with hydrogen several times, add hydrogen to the pressure of 2.0Mpa in the kettle, react at 40°C for 4h, filter the reaction solution, recover and reuse palladium carbon, add 75L ethanol to the filtrate, stir and crystallize at 0~5°C for 5h , 0.40Kg of doripenem was suction filtered, the molar yield was 70.0%, the HPLC purity was 98.2%, and the heavy metal content was <10ppm.
Embodiment 3
[0045] Embodiment 3: Preparation of doripenem
[0046] Add 1.0Kg (1.09mol) of doripenem intermediate 3, 10% Pd / CO 0.5Kg, 0.32L (5.45mol) of 3,5-lutidine, 15L of deionized water, and nitrogen into a 50L hydrogenation kettle. Substitute several times, replace with hydrogen several times, add hydrogen to the pressure of 1.5Mpa in the kettle, react at -10°C for 5h, filter the reaction solution, recover and reuse palladium carbon, add 45L ethanol to the filtrate, stir and crystallize at 0-5°C After 5 hours, 0.31Kg of doripenem was filtered with suction, the molar yield was 67.7%, the HPLC purity was 98.5%, and the heavy metal content was <10ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com