Method for circularly removing impurity chlorine in zinc sulfate solution by using cuprous salt
A zinc sulfate and cuprous technology, which is applied in the direction of copper chloride, copper halide, process efficiency improvement, etc., can solve the problem of zinc sulfate solution treatment that is difficult to adapt to a large range of chlorine content, the ion exchange dechlorination method has a small application range, and solvent extraction In order to solve the problems such as difficulty in large-scale operation of the method, it can achieve the effects of easy extraction of sodium chloride, comprehensive utilization and less impurities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
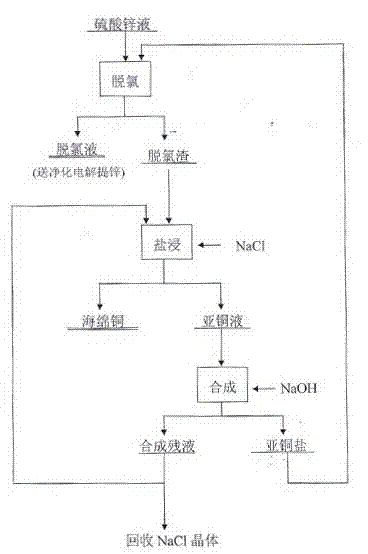
[0032] The technological process is shown in the accompanying drawing.
[0033] Dechlorination: Take 10000mL of zinc sulfate solution, the composition is (g / L): Zn 2+ 126.4 Cl - 13.78, pH5.3, put it in the reaction tank, turn on the agitator, heat up to 45°C, add 1100.0g of industrial copper sulfate, dissolve completely, adjust the pH to 2.0-2.5 with concentrated sulfuric acid, add 150.0g of zinc powder continuously, react 0.5 h, filter. The obtained filtrate composition (g / L): Zn 2+ 124.4 、Cl - 0.21, pH2.3, dechlorination rate 98.30%.
[0034] Salt leaching: Take the dechlorination residue obtained from the above dechlorination, the composition is (%): Zn3.51, Cu60.79, Cl26.75, the rest is other, put it in the reaction tank, add the - 150g / L industrial salt solution, start the agitator, heat to 70°C, adjust the pH to 2.0-2.5 with sulfuric acid, fully react for 1.5h, and filter to obtain the salt infusion and leaching residue. Leach residue (Cu29.3%, H 2 O 23.5% ), th...
Embodiment 2
[0038] The technological process is shown in the accompanying drawing.
[0039] Dechlorination: Take 10000mL of zinc sulfate solution, the composition is (g / L): Zn 2+ 132.4, Cl - 1.78, pH5.3, put it in the reaction tank, turn on the stirrer, raise the temperature to 45°C, add 1100.0g of industrial copper sulfate, dissolve completely, adjust the pH2.0~2.5 with concentrated sulfuric acid, add 150.0g of metal zinc powder, during the process Keep the pH, react for 0.5h, filter to get the filtrate, the composition is (g / L): Zn 2+ 129.4, Cl - 0.31, pH2.2, dechlorination rate 81.50%.
[0040]Salt leaching: Take the dechlorination residue obtained from the above dechlorination, the composition is (%): Zn2.51, Cu58.89, Cl 25.75, the balance is other, put it in the reaction tank, add the - 150g / L industrial salt solution, start the agitator, heat up to 70°C, adjust the pH to 2.0-2.5 with sulfuric acid, react for 1.5h, filter to obtain leaching residue, the composition is (%): Cu28....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com