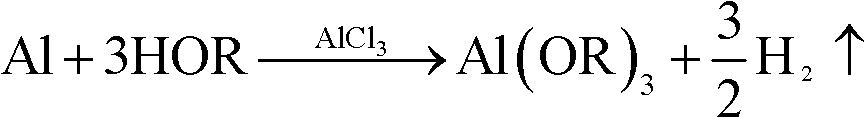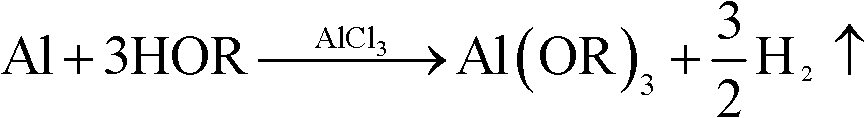Preparation of high-purity aluminum alkoxide and aluminum oxide powder from aluminium (alloy) scrap
A waste aluminum alloy and aluminum alkoxide technology, which is applied in the fields of aluminum oxide/hydroxide, organic chemistry, addition of unsaturated hydrocarbons and saturated hydrocarbons, etc., to achieve the effect of simple equipment and mild production process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The first step: weigh anhydrous aluminum chloride (AlCl 3 ) 2 grams, added to 500 ml of isopropanol Put it in a heatable container (generally made of glass or stainless steel) and stir it, then add waste aluminum alloy flakes or chips - containing 2 moles or 54 grams of aluminum, put it into the reflux device to heat and reflux, and the reaction starts. As shown in the following chemical reaction equation:
[0041]
[0042] After refluxing for 20-50 hours, the evolution of hydrogen stops, indicating that the synthesis reaction is complete. If the alloying element Mg is present, the following reactions will be accompanied at the same time:
[0043]
[0044] The second step: after the alkoxide obtained in the first step is cooled, alkoxide crystals are precipitated, the residue sinks, and the clear saturated solution of alkoxide is located at the uppermost layer. Pour the clear liquid into another clean container, add 500 ml of isopropanol to the original contai...
Embodiment 2
[0048] The first step: weigh anhydrous aluminum chloride (AlCl 3 ) 2 grams, added to a heatable container (generally made of glass or stainless steel) containing 500 milliliters of isobutanol and stirred, then added scrap aluminum alloy pieces or chips - containing 2 moles or 54 grams of aluminum, put The reflux device is heated to reflux, and the reaction begins. As shown in the following chemical reaction equation:
[0049]
[0050] After refluxing for 10 to 30 hours, the evolution of hydrogen stops, indicating that the synthesis reaction is complete. If the alloying element Mg is present, the following reactions will be accompanied at the same time:
[0051]
[0052] The second step: after the alkoxide obtained in the first step is cooled, alkoxide crystals are precipitated, the residue sinks, and the clear saturated solution of alkoxide is located at the uppermost layer. Pour the clear liquid into another clean container, add 500 ml of isobutanol to the original c...
Embodiment 3
[0057] The first step: weigh anhydrous aluminum chloride (AlCl 3 ) 2 grams, added to a heatable container (generally glass or stainless steel) containing 500 ml of n-butanol and stirred, then added scrap aluminum (alloy) pieces or chips - containing 2 moles of aluminum or 54 grams, Put into the reflux device and heat to reflux, and the reaction starts. As shown in the following chemical reaction equation:
[0058]
[0059] After reflux for 4 to 10 hours, the evolution of hydrogen stops, indicating that the synthesis reaction is complete. If the alloying element Mg is present, the following reactions will be accompanied at the same time:
[0060]
[0061] The second step: after the alkoxide obtained in the first step is cooled, alkoxide crystals are precipitated, the residue sinks, and the clear saturated solution of alkoxide is located at the uppermost layer. Pour the clear liquid into another clean container, add 500 ml of n-butanol to the original container, heat to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com