Levofloxacin hydrochloride crystal forms and preparation methods thereof
A technology of levofloxacin hydrochloride and crystal form, which is applied in the field of medicinal chemistry and achieves the effects of good fluidity, great application value and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Preparation of levofloxacin hydrochloride crystal form I
[0047]Method A: Weigh 5g of levofloxacin hydrochloride sample, put it into a round bottom flask, add 25mL of 75% ethanol aqueous solution, heat to 70°C and reflux until completely dissolved. After natural cooling, white crystals precipitated, continued cooling, and the crystallization stopped at 20°C. Suction filtration, drying at 30-40°C.
[0048] Method B: Weigh 10 g of levofloxacin hydrochloride sample, put it into a round bottom flask, add 100 mL of 50% isopropanol aqueous solution, heat to 70° C. and reflux until completely dissolved. Cool in an ice-water bath, white crystals precipitate out, continue to cool, and stop crystallization at 20°C. Suction filtration, drying at 30-40°C.
[0049] Method C: Weigh 5g of levofloxacin hydrochloride sample, put it into a round bottom flask, add 30mL of 80% aqueous methanol solution, heat to 60°C and reflux until completely dissolved. Natural cooling,...
Embodiment 2
[0050] Embodiment 2: preparation method of levofloxacin hydrochloride crystal form II
[0051] Method A: Weigh 2g of levofloxacin hydrochloride sample, put it into a round bottom flask, add 40mL of methanol solution, heat to 60°C and reflux until completely dissolved. After natural cooling, yellowish crystals precipitated, continued cooling, and the crystallization stopped at 20°C. Suction filtration, drying at 80-90°C.
[0052] Method B: Weigh 2 g of levofloxacin hydrochloride sample, add it to a round bottom flask, add 40 mL of methanol and ethanol mixed solution (the ratio of methanol to ethanol is 3:1), heat to 60 ° C and reflux until completely dissolved. After natural cooling, yellowish crystals precipitated, and the cooling continued until the crystallization stopped at 20°C. Suction filtration, drying at 80-90°C.
[0053] Method C: Weigh 10 g of levofloxacin hydrochloride sample, add it to a round bottom flask, add 240 mL of a mixed solution of methanol and acetone ...
Embodiment 3
[0055] Embodiment 3: preparation method of levofloxacin hydrochloride crystal form III
[0056] Method A: Weigh 2g of sample, put it into a round bottom flask, add 4.5mL of 60% isopropanol, heat to 75°C and reflux until completely dissolved. After natural cooling, white crystals precipitated, cooled to room temperature, and continued to stir for 30 minutes to stop the crystallization. Suction filtration, drying at 60-70°C.
[0057] Method B: Weigh 5g of sample, put it into a round bottom flask, add 25mL of 75% ethanol, heat to 75°C and reflux until completely dissolved. Cool naturally, white crystals precipitate, cool to room temperature, continue to stir for 30 minutes, stop crystallization and suction filtration, and dry at 60-70°C.
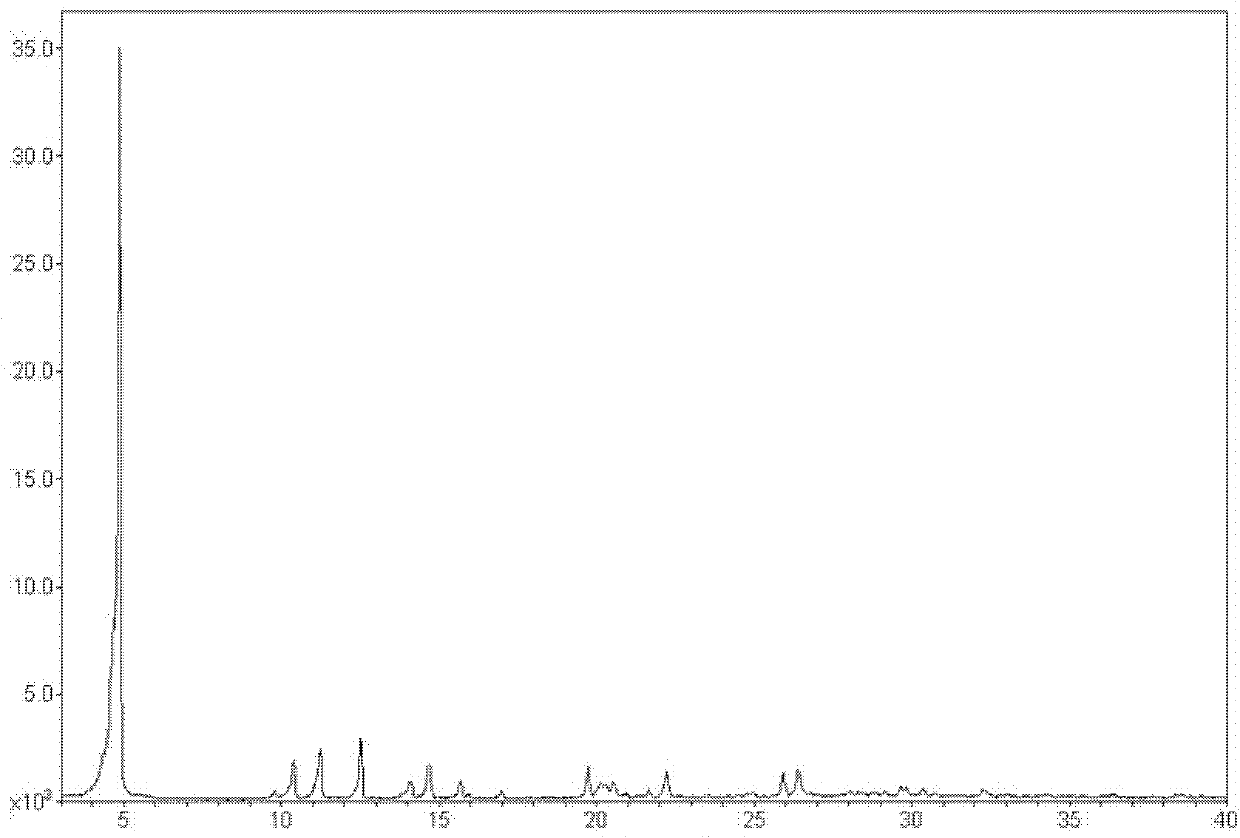
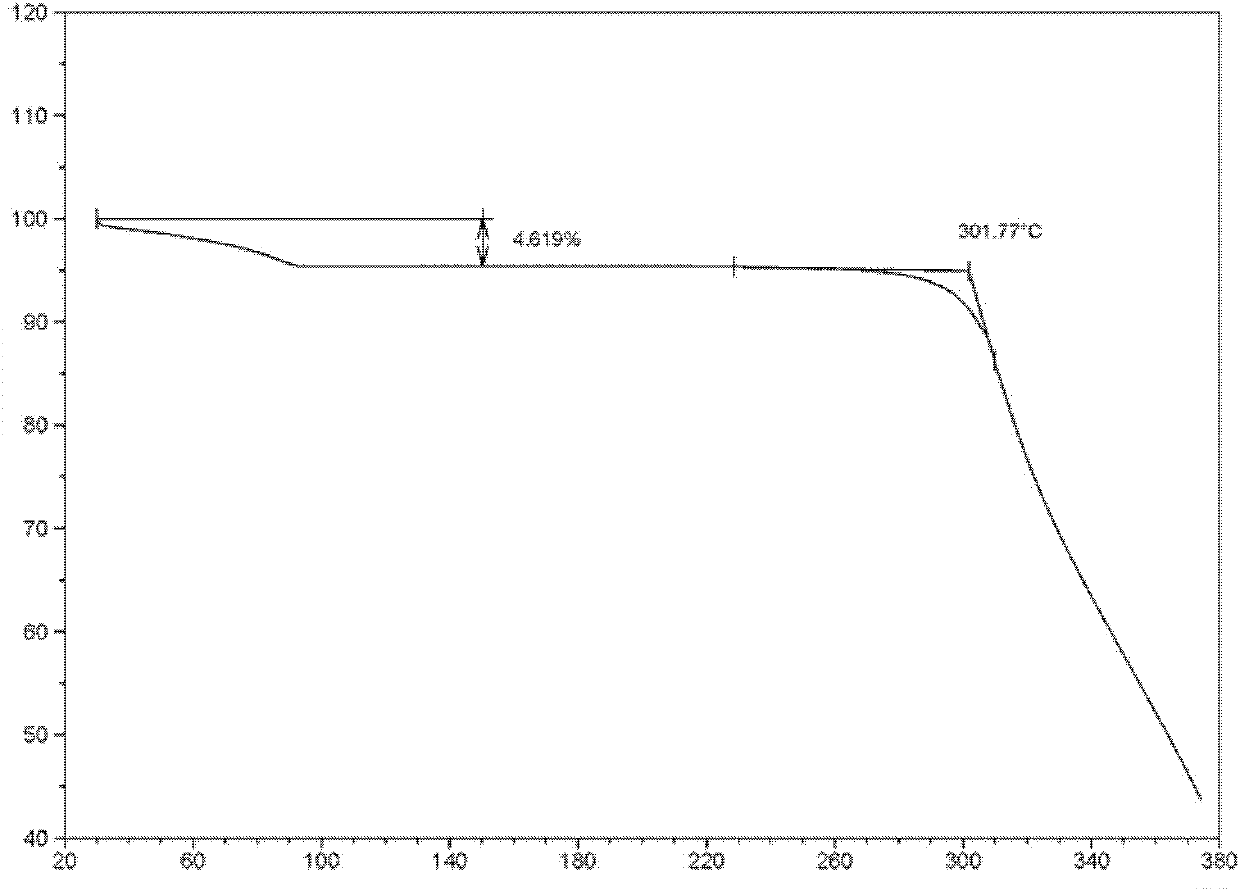
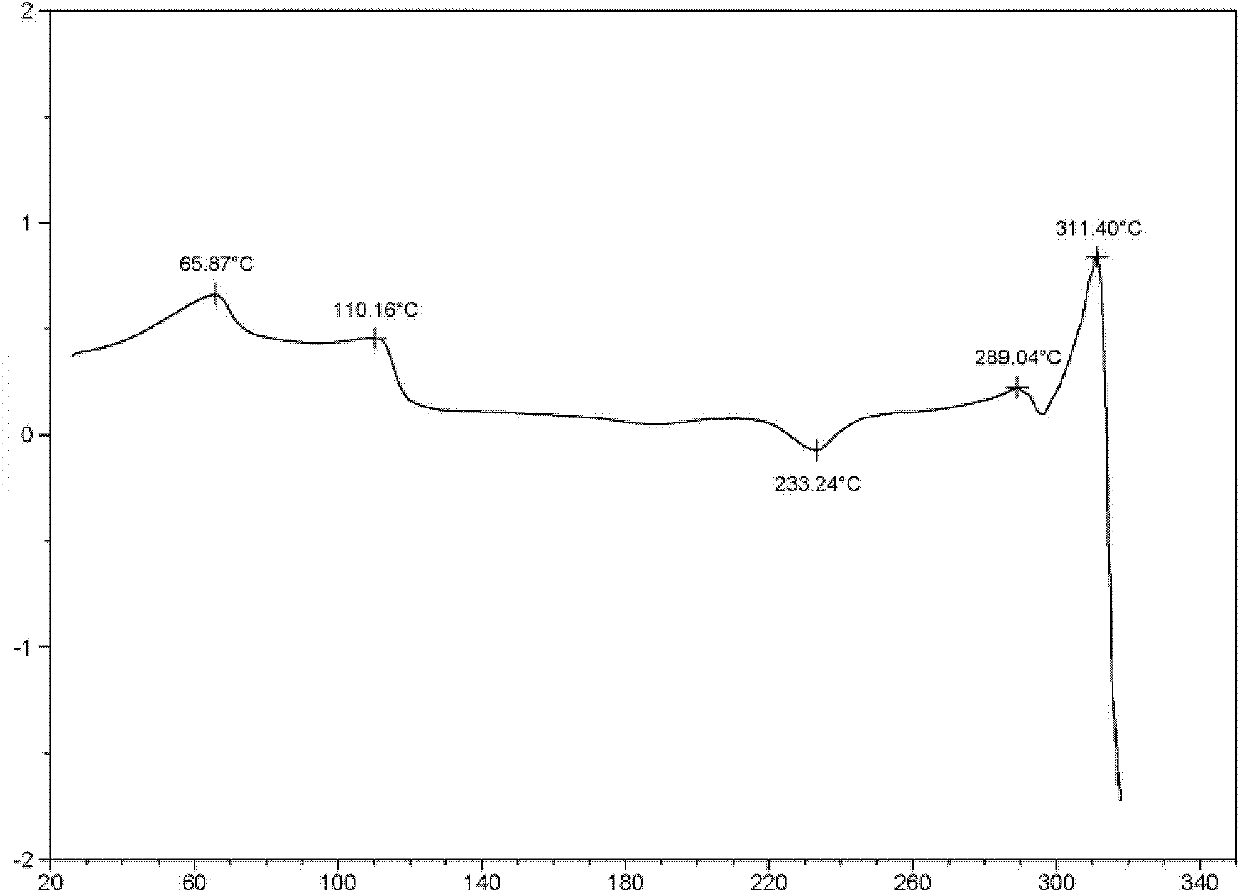
[0058] The structural characterization of levofloxacin hydrochloride crystal forms I, II, and III prepared by the above method is as follows:
[0059] The structural characterization of the levofloxacin hydrochloride crystal form I prepared ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com