Anti-fatty acid synthetase polypeptide and its application
A fatty liver and amino acid technology, applied in the field of fatty acid synthase-resistant polypeptides, can solve the problems of unstable chemical properties, strong toxic side effects, etc., and achieve the effects of no immunogenicity, good water solubility, and obvious pharmacodynamic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Synthetic polypeptide functional fragment Anti-FAS-P18:
[0089] The present invention has synthesized the amino acid sequence of Gly-Gly-Cys-Arg-His-Lys-Leu-Val-Cys-Ala-Pro-Ala-Pro-Cys-Asn-Phe-Phe-Thr (SEQ ID NO: 1) polypeptide (hereinafter referred to as Anti-FAS-P18). The preparation of the polypeptide adopts a solid-phase synthesis method, such as using an AAPPTECAPex396 polypeptide synthesis instrument (purchased from Hong Kong Universal Analytical and Testing Instrument Co., Ltd.), in a closed explosion-proof glass reactor to make amino acids according to the sequence shown in SEQ ID NO: 1, From C-terminus-carboxyl-terminus to N-terminus-amino-terminus, this refers to the first amino acid sequence Gly-Gly-Cys-Arg-His-Lys-Leu-Val-Cys-Ala-Pro-Ala-Pro to be added to the amino acid sequence -The amino acid monomer of Cys-Asn-Phe-Phe-Thr is C-terminal Thr, then Phe, then Phe, until the last Gly and N-terminal Gly, continuously adding, reacting, synthesizing, operating...
Embodiment 2
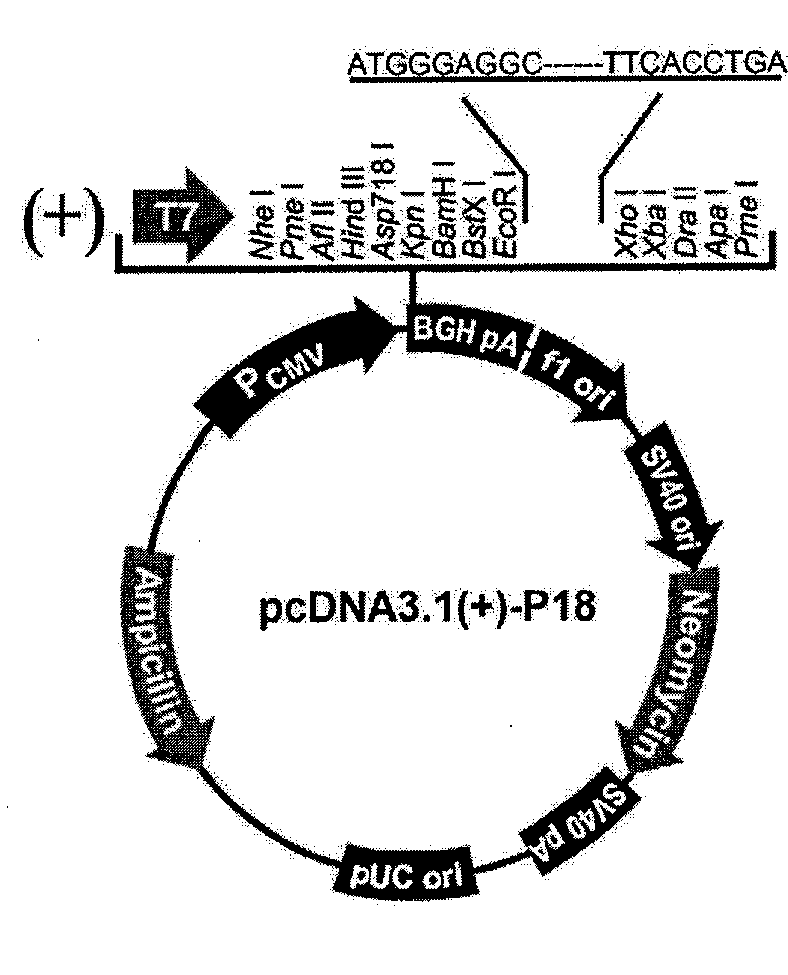
[0097] Two methods are used to detect the anti-FAS activity of the polypeptide in Example 1 in vitro: the first method is to use molecular cloning technology to clone the cDNA expressing the polypeptide in Example 1 into the eukaryotic expression vector pcDNA3.1 On (+), through gene transfection, the purpose of expressing the studied polypeptide in liver cancer cells is achieved, and then the effect of the studied polypeptide on inhibiting FAS is observed; the second method is to use artificially synthesized polypeptides and directly add them to cultured liver cancer cells In the culture medium, observe the effect of polypeptide on inhibiting FAS.
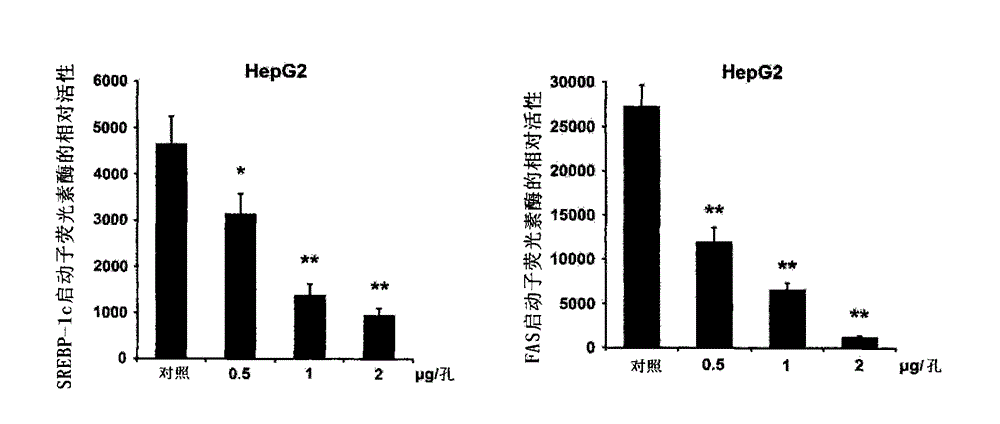
[0098] The cells used in the experiment were liver cancer HepG2 cells. Since SREBP-1c is an upstream transcriptional regulator of FAS, by luciferase reporter gene detection method, detecting whether the promoter activity of SREBP-1c is inhibited at the molecular level can reflect the gene transcription of FAS. At the same time, th...
Embodiment 3
[0213] The HepG2 cells in the logarithmic growth phase were digested with trypsin to make a cell suspension, the number of cells was calculated, and the cells were diluted to 1×10 with sterile normal saline. 7 cells / ml and stored in ice water. Twelve 4- to 6-week-old female BALB / C nude mice were then randomly divided into 2 groups: ① Control group, subcutaneously inject 0.2ml of the above-mentioned diluted cells in the armpit of the right forelimb of each mouse, and only inject 0.5 ml sterilized distilled water (without polypeptide drugs); ② experimental group (administration dose is 10 mg / kg body weight). Subcutaneously inject 0.2ml of the above-mentioned diluted cells into the armpit of the right forelimb of each mouse, and after 7 days of injection, the tumor volume (V=L×W 2 ×0.5) up to 100mm 3 , and then subcutaneously inject the above polypeptide drugs (dissolve the freeze-dried polypeptide drugs with 0.5ml sterilized distilled water), inject once every two days, and in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com