Preparation method of organic-inorganic hybrid cyclodextrin chiral separation porous monolithic material
A chiral separation, monolithic material technology, applied in other chemical processes, chemical instruments and methods, etc., can solve the problems of limited chiral separation ability, limited dose of chiral selection, poor mechanical properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
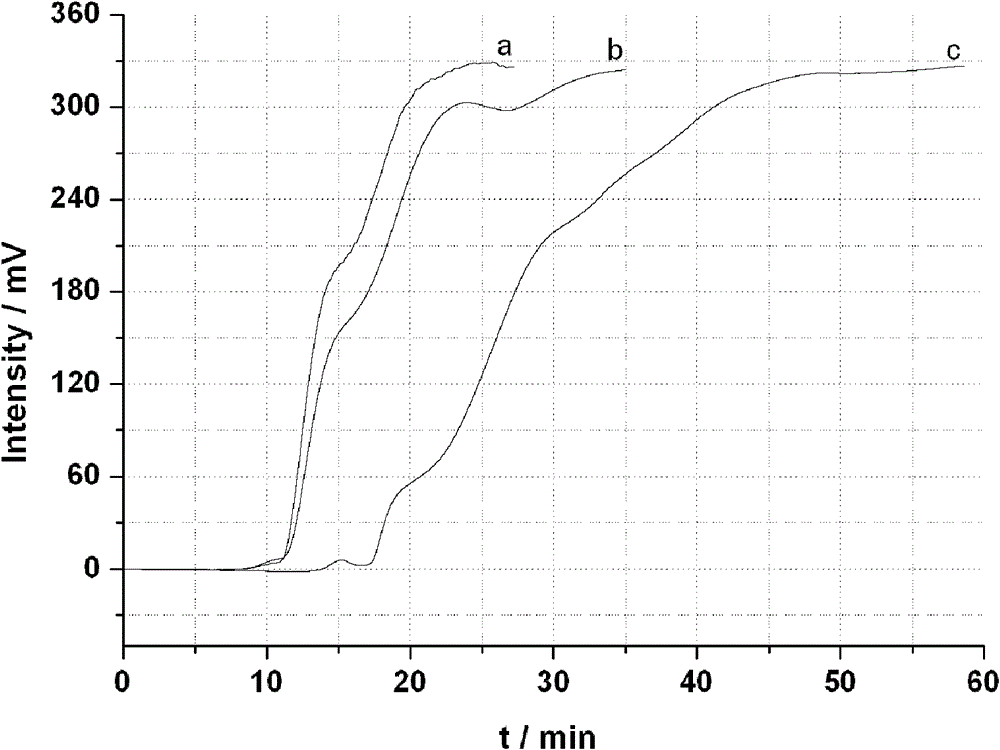
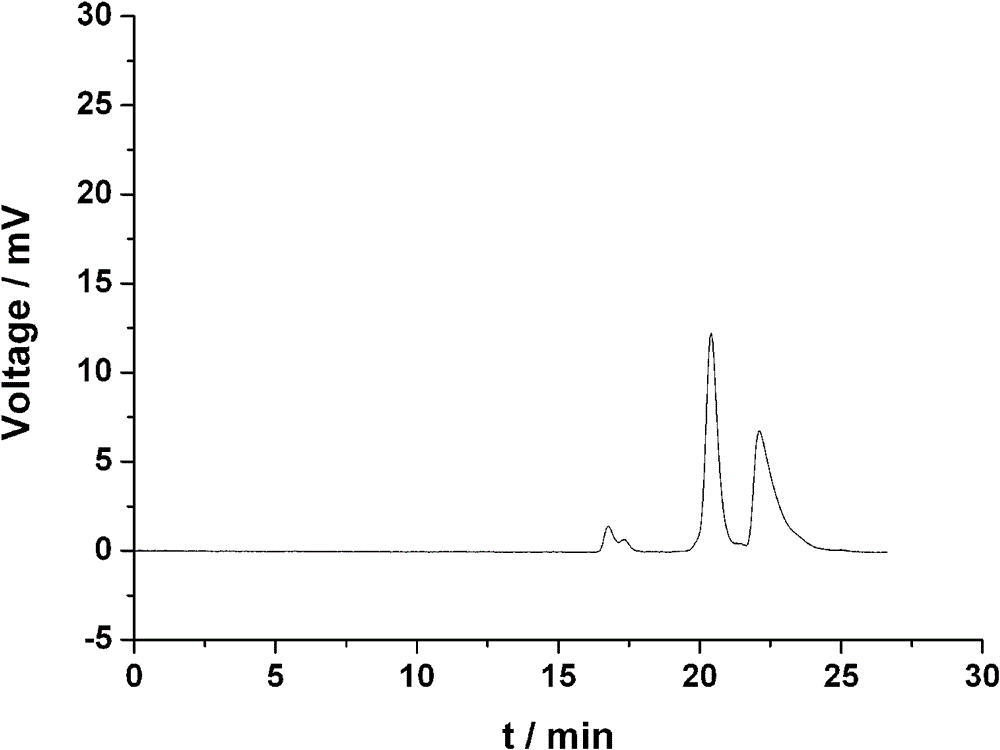
Image
Examples
Embodiment 1
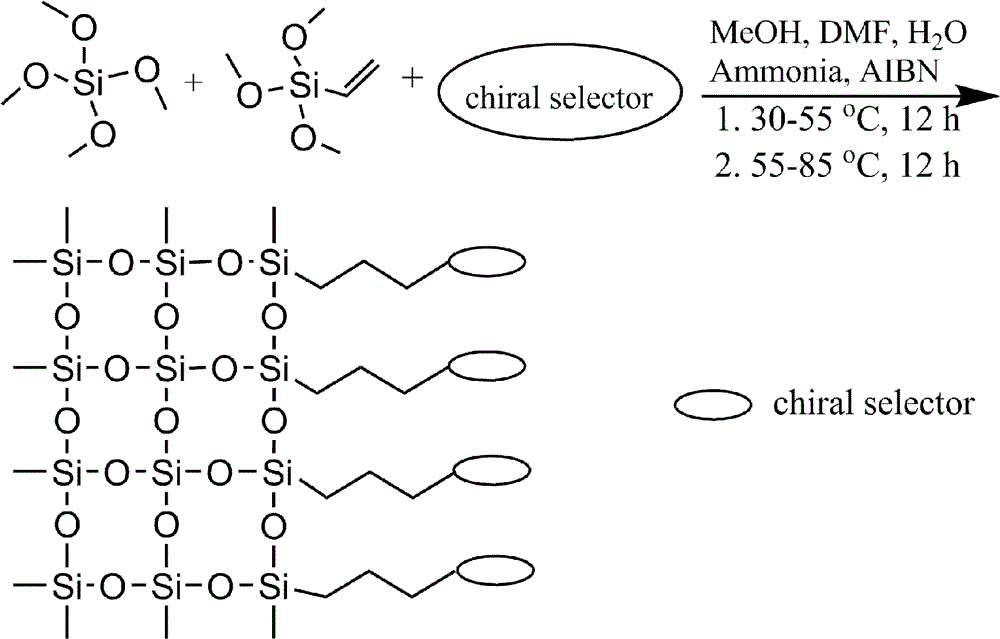
[0026] 1. Add 5 mg cetyltrimethylammonium bromide to 60 μL DMF, 190 μL MeOH, 62 μL H 2 O, magnetically stirred at room temperature to dissolve it;
[0027] 2. Slowly add 150 μL VTMS and 125 μL TMOS to the above solution, and hydrolyze in an ice bath for about 1 hour to obtain a uniform and transparent hydrolyzate;
[0028] 3. Combine 20mg mono (6 A -N-allylamino-6 A -deoxy)-β-CD, 50 μL of 10 μM ammonia solution and 1 mg of azobisisobutyronitrile (AIBN) were added to the above hydrolyzate, and sonicated for 3-20 minutes to make it evenly mixed and remove oxygen;
[0029] 4. Use a syringe to introduce the pre-polymerization solution obtained in 3 into a capillary tube with an inner diameter of 75 μm that has been treated with acid and alkali. Seal the remaining pre-polymerization solution in the centrifuge tube with parafilm.
[0030] 5. Put the capillary and centrifuge tube in 4 in a water bath at a temperature of 37°C, and react for 12 hours. At this time, the pre-polymeri...
Embodiment 2
[0034] 1. Add 5 mg cetyltrimethylammonium bromide to 60 μL DMF, 190 μL MeOH, 62 μL H 2 O, magnetically stirred at room temperature to dissolve it;
[0035] 2. Slowly add 150 μL VTMS and 125 μL TMOS to the above solution, and hydrolyze in an ice bath for about 1 hour to obtain a uniform and transparent hydrolyzate;
[0036] 3. Combine 30mg mono (6 A -N-allylamino-6 A-deoxy)-perphenylcarbamoylatedβ-CD, 50 μL of 10 μM ammonia solution and 2 mg of AIBN were added to the above hydrolyzate, and ultrasonically treated for 3-20 minutes to make it evenly mixed and remove oxygen;
[0037] 4. Use a syringe to introduce the pre-polymerization solution obtained in 3 into a capillary tube with an inner diameter of 75 μm that has been treated with acid and alkali. Seal the remaining pre-polymerization solution in the centrifuge tube with parafilm.
[0038] 5. Put the capillary and centrifuge tube in 4 in a water bath at 40°C, and react for 12 hours. At this time, the pre-polymerization s...
Embodiment 3
[0042] 1. Add 5 mg cetyltrimethylammonium bromide to 60 μL DMF, 190 μL MeOH, 62 μL H 2 O, magnetically stirred at room temperature to dissolve it;
[0043] 2. Slowly add 150 μL ATMS and 125 μL TMOS to the above solution, and hydrolyze in an ice bath for about 1 hour to obtain a uniform and transparent hydrolyzate;
[0044] 3. Combine 60mg mono (6 A -N-allylamino-6 A -deoxy)-per(p-chlorophenylcarbamo-ylated)β-CD, 50 μL of 10 μM ammonia solution and 3 mg of AIBN were added to the above hydrolyzate, and ultrasonically treated for 3-20 minutes to make it evenly mixed and remove oxygen;
[0045] 4. Use a syringe to introduce the pre-polymerization solution obtained in 3 into a capillary tube with an inner diameter of 75 μm that has been treated with acid and alkali. Seal the remaining pre-polymerization solution in the centrifuge tube with parafilm.
[0046] 5. Place the capillary and centrifuge tube in 4 in a water bath at 45°C, and react for 12 hours. At this time, the pre-po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com