Nonionic hydrophilic monomer for synthesis of waterborne polyurethane and synthetic method thereof
A non-ionic hydrophilic, water-based polyurethane technology, applied in the polymer field, to facilitate large-scale industrial production, avoid emulsion breaking, and easily obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
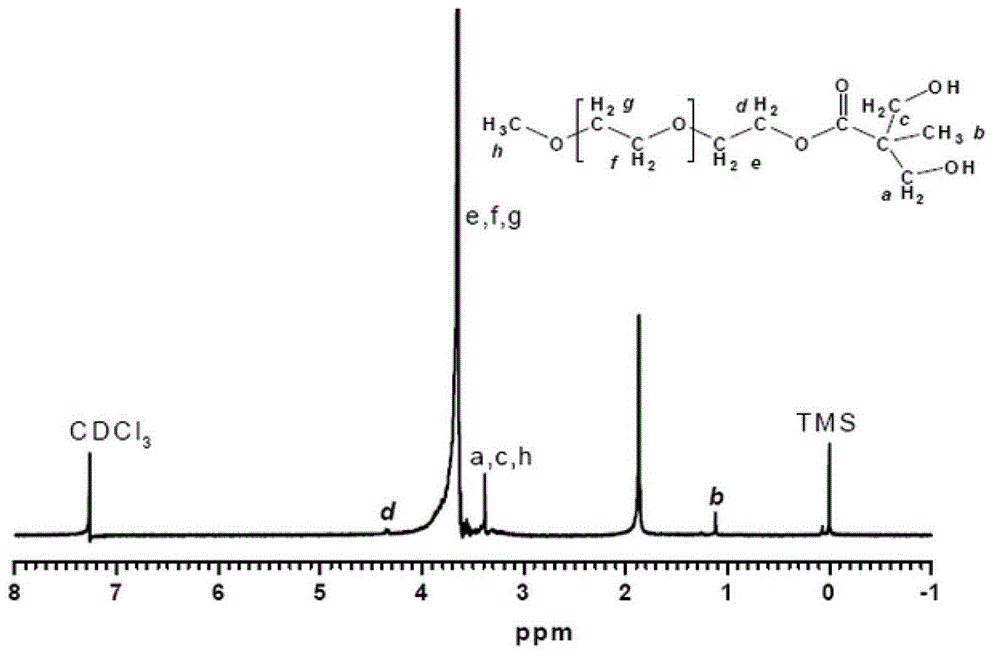
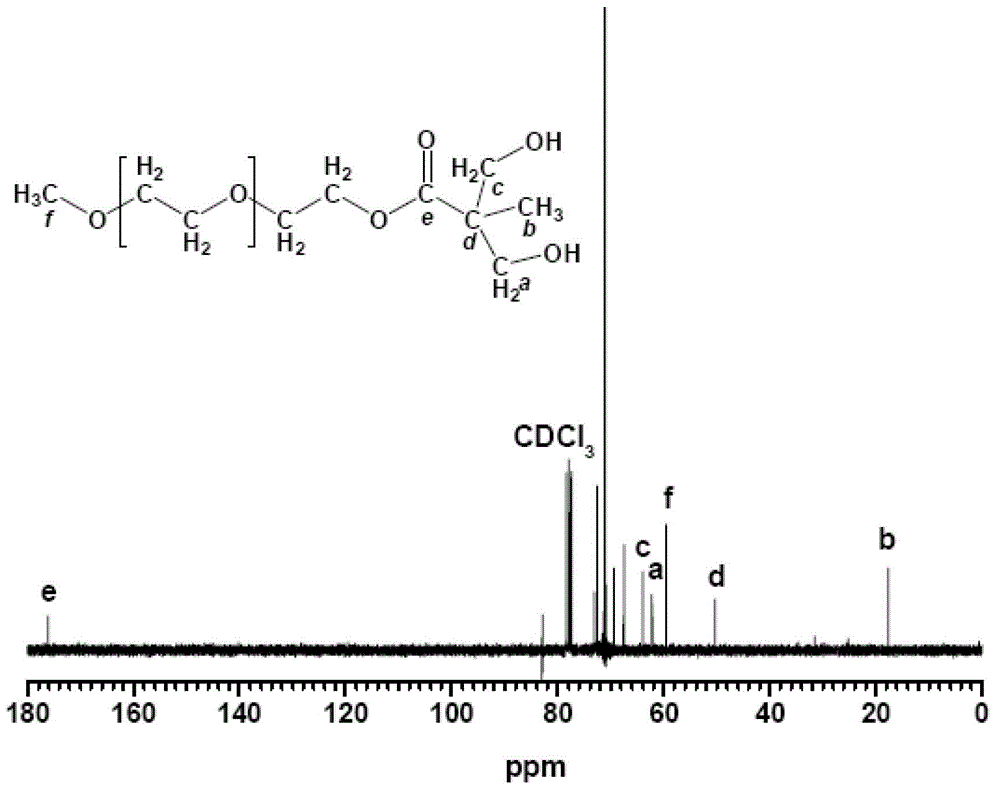
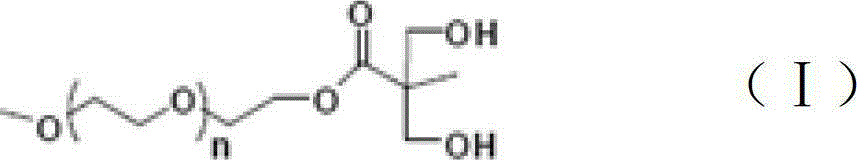
[0022] Dissolve 1mol of 2,2-dimethylolpropionic acid in 500ml of acetone, and dissolve 0.1mol of p-toluenesulfonic acid and 1.2mol of 2,2-dimethoxypropane in acetone. The above solution was put into a three-necked flask, filled with nitrogen for 30 minutes, and reacted at 60° C. for 12 hours. The reaction product was filtered out through a silica gel column, dried under vacuum for 12 hours, and the solvent was fully evaporated to obtain 170 g of the product.
[0023] Add 1700g of N,N'-dicyclohexylcarbodiimide to a three-necked flask, dissolve 170g of the above product in it, then add 0.1mol of 4-dimethylaminopyridine, and then add 1500g of polyethylene glycol monomethanol with a molecular weight of 1000 Ether, the esterification reaction is carried out at 120°C. After reacting for 6 hours, the product was filtered out through a silica gel column and dried for 12 hours to obtain 1660 g of an esterified product.
[0024] Add 840g of 37% hydrochloric acid and 840g of methanol i...
Embodiment 2
[0027] Dissolve 1.2mol of 2,2-dimethylolpropionic acid in 500ml of acetone, and dissolve 0.12mol of p-toluenesulfonic acid and 2mol of 2,2-dimethoxypropane in acetone. The above solution was put into a three-necked flask, filled with nitrogen for 30 minutes, and reacted at 60° C. for 10 hours. The reaction product was filtered out through a silica gel column, dried under vacuum for 12 hours, and the solvent was fully evaporated to obtain 204 g of the product.
[0028] Add 2000g of N,N'-dicyclohexylcarbodiimide to a three-necked flask, dissolve 204g of the above product in it, then add 0.12mol of 4-dimethylaminopyridine, and then add 1800g of polyethylene glycol monomethyl ether with a molecular weight of 1000 , The esterification reaction was carried out at 130°C. After reacting for 6 hours, the product was filtered out through a silica gel column and dried for 12 hours to obtain 2000 g of the esterified product.
[0029] Add 1000g of 37% concentrated hydrochloric acid and 1...
Embodiment 3
[0031] Dissolve 2mol 2,2-dimethylolpropionic acid in 1000ml dimethylformamide, and dissolve 0.2mol p-toluenesulfonic acid and 3mol 2,2-dimethoxypropane in dimethylformamide amides. The above solution was put into a three-necked flask, filled with nitrogen for 30 minutes, and reacted at 60° C. for 10 hours. The reaction product was filtered out through a silica gel column, dried under vacuum for 12 hours, and the solvent was fully evaporated to obtain 337 g of the product.
[0032] Add 3200g of N,N'-dicyclohexylcarbodiimide to a three-necked flask, dissolve 337g of the above product in it, then add 0.2mol of 4-dimethylaminopyridine, and then add 2700g of polyethylene glycol monomethyl ether with a molecular weight of 1000 , The esterification reaction was carried out at 130°C. After reacting for 6 hours, the product was filtered out through a silica gel column and dried for 12 hours to obtain 3000 g of an esterified product.
[0033] Add 2000g of 37% concentrated hydrochlori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com