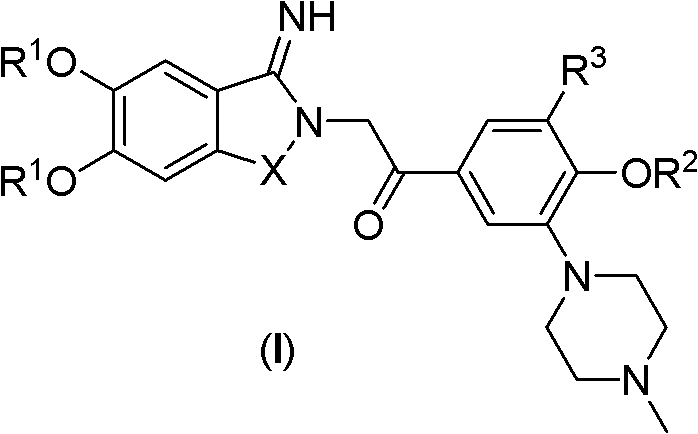
Exocyclic imine compound containing benzo five-membered heterocycle, preparation method and application of exocyclic imine compound
A compound and drug technology, applied in the field of drugs related to thrombotic diseases, can solve problems such as high bleeding risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
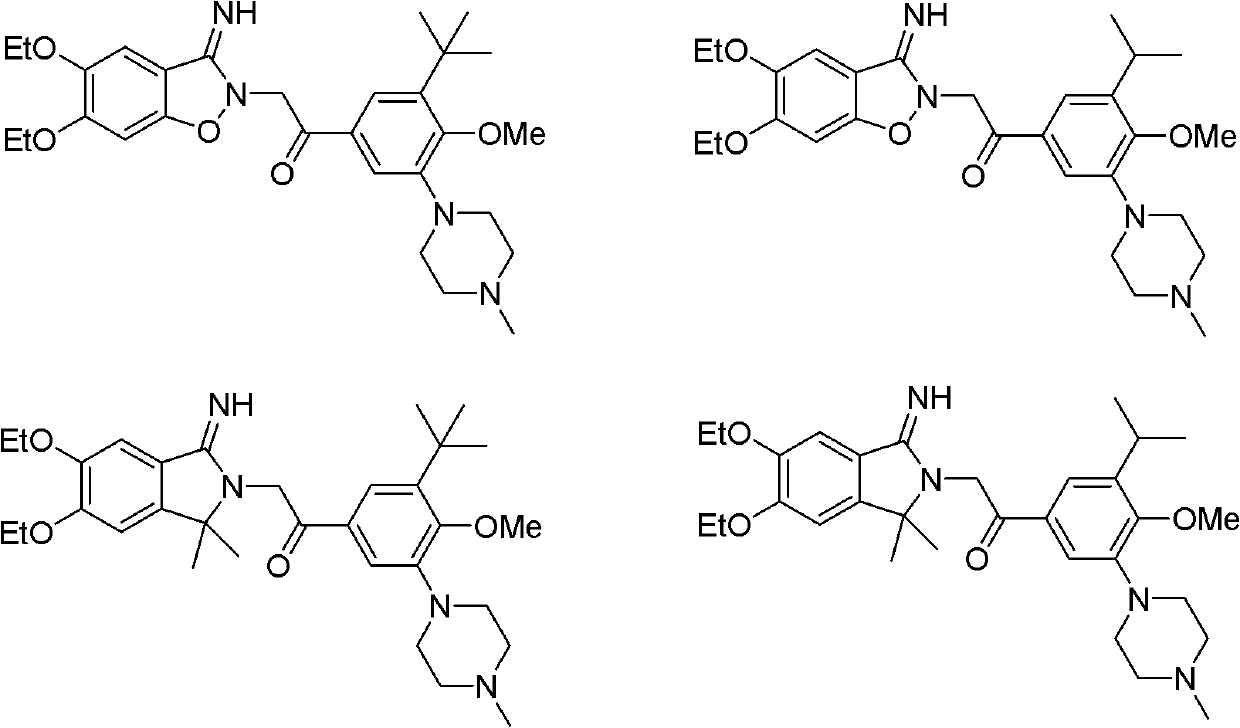
[0037] Example 1 1-[3-tert-butyl-4-methoxy-5-(4-methylpiperazin-1-yl)phenyl]-2-(5,6-diethoxy-2,3-di Hydrogen-3-iminobenzo[d]isoxazol-2-yl)ethanone hydrobromide
[0038]
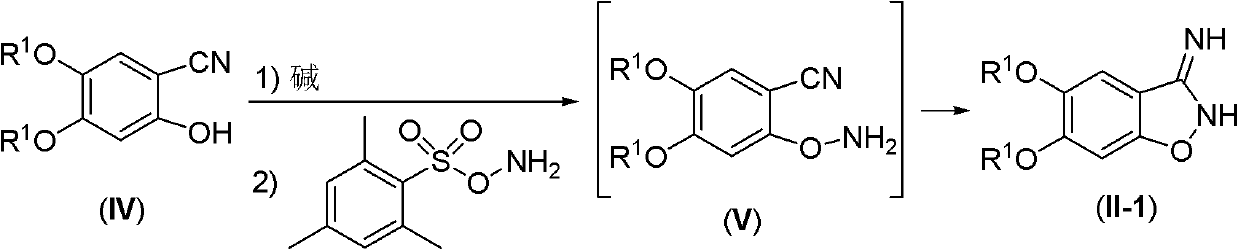
[0039] A. 5,6-diethoxy-2,3-dihydro-3-iminobenzo[d]isoxazole
[0040]
[0041] A 50mL round bottom flask was added with 2.07g (10mmol) of 4,5-diethoxy-2-hydroxybenzonitrile and 20mL of anhydrous methanol, the resulting mixture was stirred at room temperature, and then 0.56g (10mmol) of solid KOH was added , continued to stir for 5 minutes and then evaporated to dryness on a rotary evaporator, and the obtained residue was dried at room temperature on a vacuum oil pump for 1 hour. To the obtained solid was added 10 mL of dry DMF, stirred at room temperature, and then O-(2,4,6-trimethylbenzenesulfonyl)hydroxylamine was added, and the resulting mixture was stirred overnight at room temperature. TLC showed the reaction was complete.
[0042] The reaction mixture was cooled and poured into 200 mL of ice w...
Embodiment 2
[0045] Example 2 1-[3-isopropyl-4-methoxy-5-(4-methylpiperazin-1-yl)phenyl]-2-(5,6-diethoxy-2,3-di Hydrogen-3-iminobenzo[d]isoxazol-2-yl)ethanone hydrobromide
[0046]
[0047] In a 50mL round-bottomed flask, add 1.56g (7mmol) of 5,6-diethoxy-2,3-dihydro-3-iminobenzo[d]isoxan prepared above according to Example 1 oxazole and 2.59 g (7 mmol) of 3-isopropyl-4-methoxy-5-(4-methylpiperazin-1-yl)-ω-bromoacetophenone were dissolved in 20 mL of dry THF. The resulting mixture was stirred overnight at room temperature, and the resulting yellow cloudy system was filtered with suction, and the solid was collected and washed with a small amount of dry THF. The obtained filter cake was vacuum-dried at room temperature to obtain 1-[3-isopropyl-4-methoxy-5-(4-methylpiperazin-1-yl)phenyl]-2-(5, 6-diethoxy-2,3-dihydro-3-iminobenzo[d]isoxazol-2-yl)ethanone hydrobromide, off-white solid. ESI-MS, m / z=511 ([M+H] + ).
Embodiment 3
[0048] Example 3 1-[3-tert-butyl-4-methoxy-5-(4-methylpiperazin-1-yl)phenyl]-2-(5,6-diethoxy-1,3-di Hydrogen-1,1-dimethyl-3-imino-2H-isoindol-2-yl)ethanone hydrobromide
[0049]
[0050] A. 4,5-diethoxy-2-(1-methylvinyl)benzonitrile
[0051]
[0052] A 100 mL round bottom flask was charged with 2.85 g (10 mmol) of 1-bromo-2-(1-methylvinyl)-4,5-diethoxybenzene, 1.79 g (20 mmol) of CuCN and 30 mL of dry DMF , and the resulting mixture was stirred at reflux for 5 hours under nitrogen atmosphere. TLC showed the reaction was complete.
[0053] The reaction mixture was slowly poured into 200 mL of ice water, stirred, extracted with 50 mL×3 dichloromethane, and the extracted organic phases were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, and the obtained filtrate was evaporated to remove the solvent on a rotary evaporator, and the obtained residue was purified by column chromatography to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com