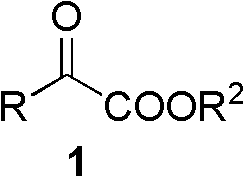
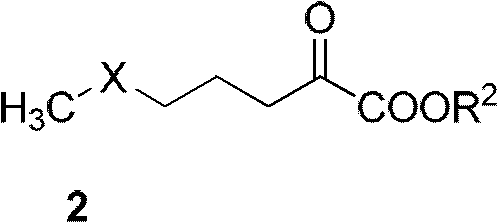
Synthetic method of alpha-amino acid with photolytic activity
A synthetic method and photoactive technology, which are applied in the field of α-amino acid synthesis, can solve the problems of waste of raw materials, impact on application, limitation of chiral α-amino acid species, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 (R)-2-amino-4-phenyl-butyric acid-3-ethyl-3-pentyl ester (see structural formula 12-a):
[0070]
[0071] n Bu is n-butyl; Et is ethyl; Cat represents catalyst
[0072] Under the protection of nitrogen, add 1-phenyl-3-oxobutanoic acid-3-ethyl-3-pentyl ester (0.50mmol, 0.138g) of the structural formula 5-a described in the above reaction formula to the reactor successively , o-chlorobenzylamine (0.50 mmol, 0.071 g) and 1.0 mL of benzene. Put the reactor into an oil bath at 70° C. After reacting for 12 hours, take the reactor out from the oil bath and place it at room temperature, and add the chiral base (0.05 mmol, 0.018g) and 9.0mL benzene, adjust the temperature of the oil bath to 50°C. After 24 hours, the heating was stopped, and the solvent benzene was spin-dried to obtain the crude transamination product of the structural formula 11-a described in the above reaction formula. The conversion rate was 100% as determined by NMR, and the next step reactio...
Embodiment 2
[0077] Embodiment 2 (R)-2-amino-4-phenylbutyric acid ethyl ester (see structural formula 12-b):
[0078]
[0079] Et is ethyl; Cat is catalyst
[0080] Under the protection of nitrogen, add 1-phenyl-3-oxobutanoic acid ethyl (0.20mmol, 0.041g), p-fluorobenzylamine (0.50mmol , 0.063g) and 1.0mL toluene. Put the reactor into an oil bath at 25° C. After reacting for 2 hours, take the reactor out from the oil bath and place it at room temperature, and add the chiral base (0.02 mmol, 0.006g) and 1.0mL toluene, adjust the temperature of the oil bath to 100°C. Heating was stopped after 15 hours, and the crude product of transamination with the structural formula 11-b described in the above reaction formula was obtained after spin-drying toluene. The conversion rate was 100% as determined by NMR, and the next step reaction was directly performed without further purification.
[0081] The transamination crude product obtained by the above reaction with the structural formula 11-b ...
Embodiment 3
[0085] Embodiment 3 (R)-2-amino-4-phenylbutyric acid tert-butyl ester (see structural formula 12-c):
[0086]
[0087] t Bu is tert-butyl; Cat represents catalyst
[0088] Under the protection of nitrogen, add 1-phenyl-3-oxobutanoic acid tert-butyl (0.20mmol, 0.047g) of 1-phenyl-3-oxobutanoic acid tert-butyl (0.20mmol, 0.047g), o-chlorobenzylamine (0.20 mmol, 0.028g) and 0.5mL toluene. Put the reactor into an oil bath at 100° C. After reacting for 12 hours, take the reactor out from the oil bath and place it at room temperature, and add the chiral base (0.02 mmol, 0.007g) and 1.5mL toluene, the temperature of the oil bath was adjusted to 40°C. Heating was stopped after 12 hours, and the crude product of transamination with the structural formula 11-c described in the above reaction formula was obtained after spin-drying toluene. The conversion rate was 100% as determined by NMR, and the next step reaction was directly carried out without further purification.
[0089] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com