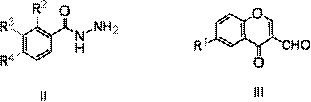
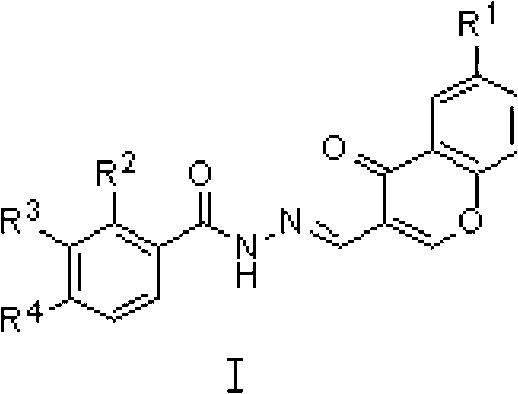
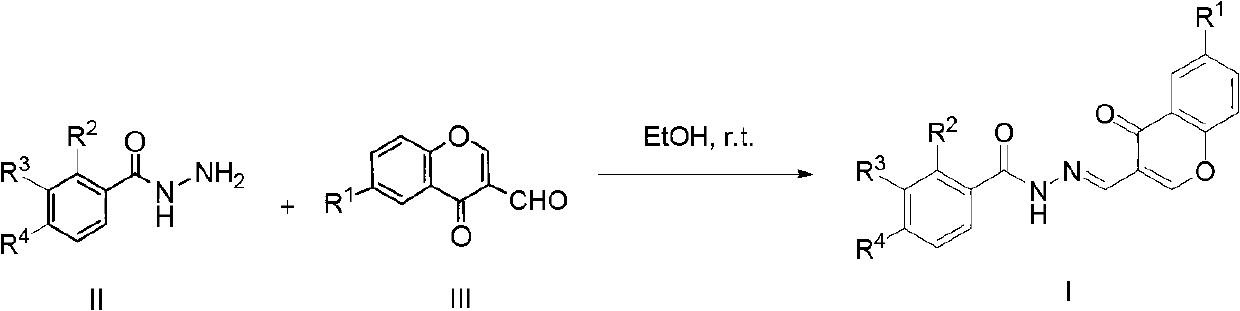
Chromone-containing benzoyl hydrazone compound capable of suppressing growth of cyanobacteria
A technology for benzoyl hydrazones and compounds, which is applied in the field of chromone-containing benzoyl hydrazone compounds and their preparation, and can solve problems such as unfavorable fish production and fish death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Compound I-1
[0014] Preparation of nitrogen'-[(4-oxo-4hydro-chromone-3-)methene]-3-bromobenzohydrazide
[0015]
[0016] Dissolve 1 mmol of 3-bromobenzoylhydrazide in 10 ml of absolute ethanol, add 1 mmol of 3-formylchromone, and stir at room temperature for 1 hour, a milky white turbidity is formed. Suction filtration, washing with ethanol, and drying of the filter cake yielded white powder nitrogen'-[(4-oxo-4hydro-chromone-3-)methene]-3-bromobenzohydrazide with a yield of 90%, m.p. 175-178°C.
[0017] Molecular formula: C 17 h 11 BrN 2 o 3 ;
[0018] 1 H NMR(600MHz,dmso)δ12.03(s,1H,NH),8.85(s,1H,2-H),8.63(s,1H,CH=N),8.21-7.39(m,8H,ArH) ;
[0019] HR-MS(ESI):m / z=371.0038, calcd for C 17 h 11 BrN 2 o 3 [M+H] + :371.0031.
[0020] Compounds 2-19 were all prepared in a similar manner to compound 1
[0021] Compound I-2
[0022] Preparation of nitrogen'-[(6-chloro-4-oxo-4hydro-chromone-3-)methene]-3-bromobenzohydrazide
[0023]
[0024] The obtain...
Embodiment 2
[0148] The growth inhibition test of Microcystis aeruginosa FACHB 912 was carried out on the 19 compounds synthesized above. Microcystis aeruginosa FACHB 912 was cultured in BG-11 (+N) liquid medium containing different concentrations of compounds in an artificial climate incubator. The temperature of the incubator is controlled at 28°C±1°C, the humidity is 60%, the light intensity is 6000lx, 12h (light): 12h (dark), vibration mode, and manual shaking 3 times a day.
[0149] The specific operation is as follows:
[0150] 1. Pre-cultivate a batch of cyanobacteria, after 4-7 days of growth to the logarithmic phase, measure OD680, and inoculate for use;
[0151] 2. Dilute the algae liquid in the logarithmic phase with fresh BG-11 (+N) medium to obtain the inoculum liquid, and control the algae cell concentration to about 1×10 6 a / mL;
[0152] 3. Weigh the compound in advance, dissolve it in DMSO, and prepare the compound with different concentrations. Add 200 μL of the dilute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com