Expression system of fusion protein from human serum albumin and interleukin-1 receptor antagonist
A technology of human serum albumin and receptor antagonist, applied in the field of protein expression system, can solve the problem of low expression level of fusion protein, and achieve the effects of low immunogenicity, improved expression level and favorable clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Construction of plasmid pPIC9-IGH, and obtaining DNA sequence capable of expressing IGH protein
[0036] Using the plasmids PGEM-T-HSA and PGEM-T-IL1ra disclosed in Patent Application No. 200810060568.7 as templates, primers IL1ra up (SEQ ID NO. 16) and IL1ra dn (SEQ ID NO. 17) were designed according to the sequences of IL1ra gene and HSA gene. ), HSAup (SEQ ID NO. 18) and HSA dn (SEQ ID NO. 19) for PCR amplification. Since the base sequence of the BamH I restriction site exactly encodes -GS-, the codon encoding the connecting peptide -GGGGS- is designed at the 5'end of IL1ra dn, and the BamH I restriction site is also designed. A BamHI restriction site is also added at the up 5'end of HSA, so that the IL1ra gene and HSA gene can be fused by BamHI restriction and ligation. The IL1ra gene amplification product was digested with Xho I and BamH I and recovered. The HSA gene amplification product was digested with BamH I and EcoR I and recovered. The empty pPIC9 pl...
Embodiment 2
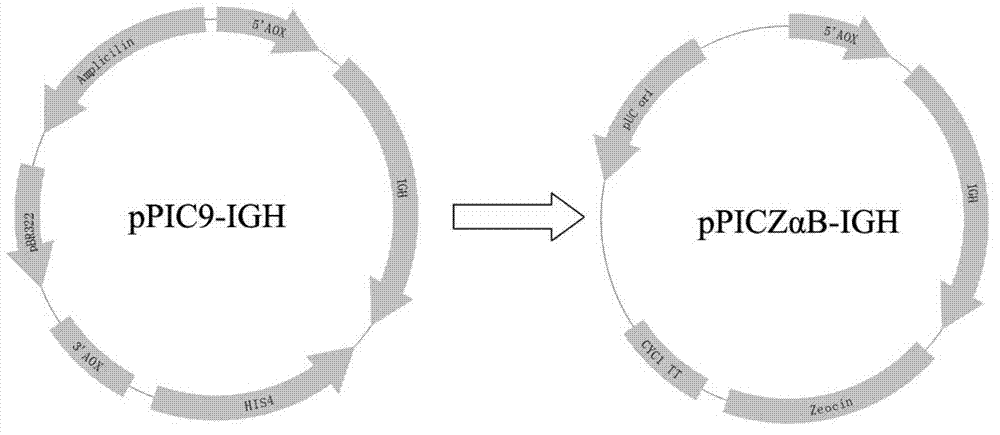
[0038] Example 2 Construction of recombinant plasmid pPICZαB-IGH
[0039] The empty pPICZαB vector was digested with Xho I and Not I, and the vector fragments were recovered by electrophoresis. The digested pPICZαB vector and the IGH expression sequence obtained in Example 1 were mixed in an appropriate ratio, and ligated with T4 ligase in a 16°C water bath for 12 hours to form pPICZαB-IGH (see the construction principle figure 1 ). After pPICZαB-IGH was transformed into DH5α, it was plated on an LB agar plate containing 25μg / mL Zeocin and incubated at 37°C overnight. Select several clones on the plate to inoculate 5mL LB liquid medium containing 25μg / mL Zeocin, and cultivate overnight at 37℃. The obtained positive clones were sent to Shanghai Shenggong for sequencing for verification.
Embodiment 3
[0040] Example 3 Transformation of GS115 by recombinant plasmid pPICZαB-IGH
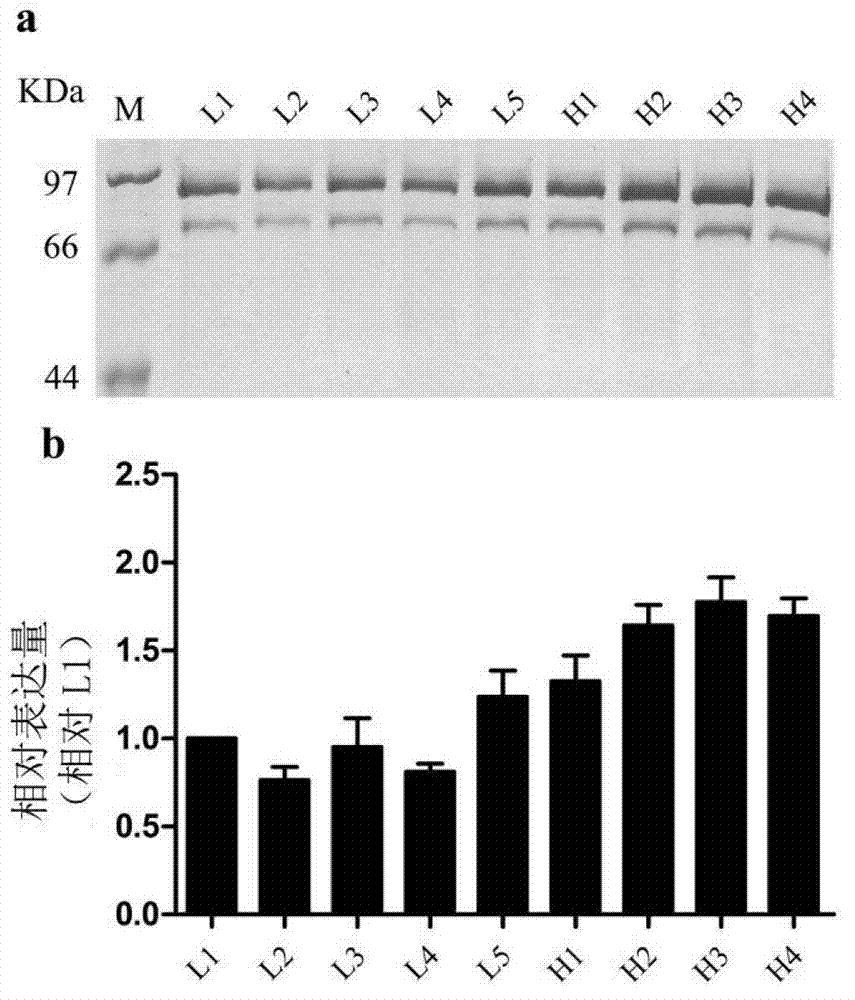
[0041] The verified pPICZαB-IGH plasmid obtained in Example 2 was linearized with Pme I and transformed into Pichia pastoris GS115 by electrotransformation. The transformed cells were incubated in YPD liquid medium at 30°C for 2 hours, and then coated To YPDS agar plates containing 100, 700 or 1500 μg / mL Zeocin (1% yeast extract, 2% peptone, 2% glucose, 1M sorbitol, 2% agarose, 1M sorbitol). After culturing at 30°C for 3 days, about 200, 4, and 0 single colonies were grown on YPDS agar plates with 100, 700 or 1500 μg / mL Zeocin. Pick 5 (named L1~5) and 4 (named H1~4) single colonies on YPDS agar plates with 100 and 700 μg / mL Zeocin, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com