Expression system of fusion protein from human serum albumin and interleukin-1 receptor antagonist
A technology of human serum albumin and receptor antagonist, applied in the field of protein expression system, can solve the problem of low expression level of fusion protein, and achieve the effects of low immunogenicity, improved expression level and favorable clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Constructing plasmid pPIC9-IGH, and obtaining the DNA sequence that can express IGH protein
[0036] Using the plasmids PGEM-T-HSA and PGEM-T-IL1ra disclosed in Patent Application No. 200810060568.7 as templates, primers IL1ra up (SEQ ID NO.16), IL1ra dn (SEQ ID NO.17) were designed according to the sequences of IL1ra gene and HSA gene ), HSAup (SEQ ID NO.18) and HSA dn (SEQ ID NO.19) were amplified by PCR. In view of the fact that the base sequence of the BamH I restriction site just encodes -GS-, the codon encoding the connecting peptide -GGGGS- is specially designed at the 5' end of IL1ra dn, so that the BamH I restriction site is also designed. And a BamHI restriction site is also added at the 5' end of HSA up, so that the IL1ra gene and the HSA gene can be fused by BamHI restriction restriction and connection. The IL1ra gene amplification product was digested with Xho I and BamH I and recovered, the HSA gene amplification product was digested with BamH I...
Embodiment 2
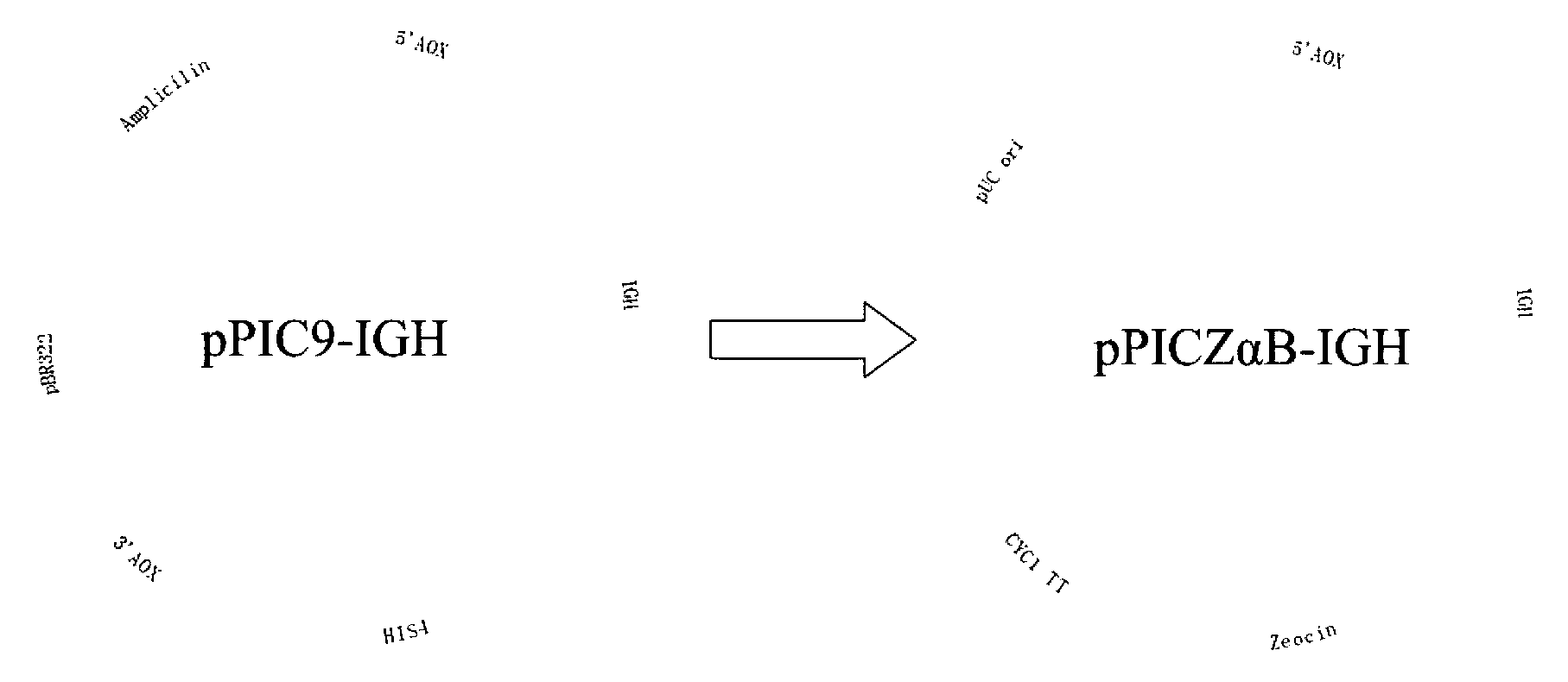
[0038] Example 2 Construction of recombinant plasmid pPICZαB-IGH
[0039] The empty pPICZαB vector was digested with Xho I and Not I, and the vector fragment was recovered by electrophoresis. The digested pPICZαB vector and the IGH expression sequence obtained in Example 1 were mixed in an appropriate ratio, and ligated with T4 ligase in a water bath at 16°C for 12 hours to form pPICZαB-IGH (see figure 1 ). After pPICZαB-IGH was transformed into DH5α, spread on LB agar plates containing 25 μg / mL Zeocin and culture overnight at 37°C. Select several clones on the plate and inoculate them into 5 mL LB liquid medium containing 25 μg / mL Zeocin, and culture overnight at 37°C. The obtained positive clones were sent to Shanghai Sangon for sequencing verification.
Embodiment 3
[0040] Example 3 Transformation of recombinant plasmid pPICZαB-IGH into GS115
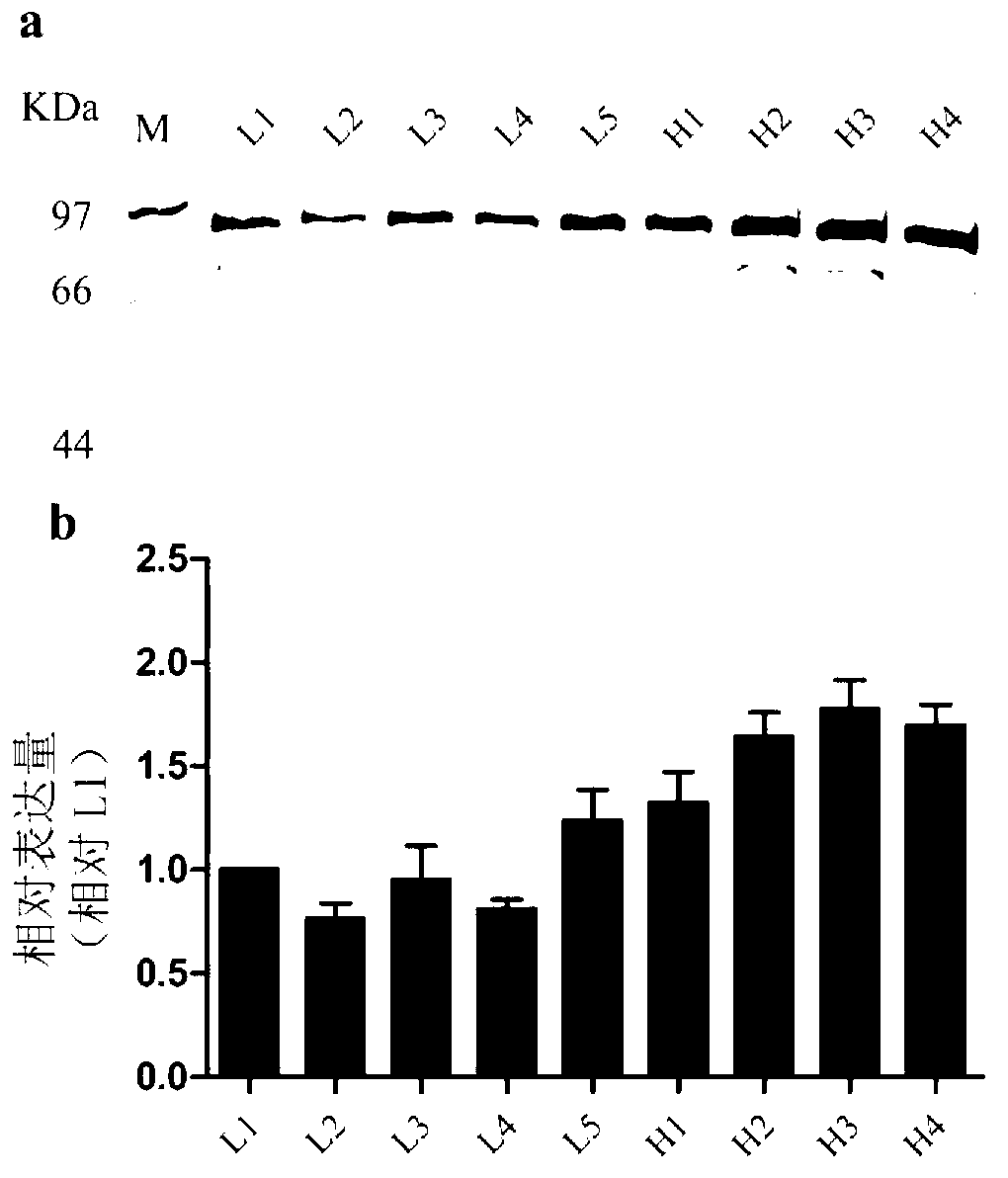
[0041] The correctly verified pPICZαB-IGH plasmid obtained in Example 2 was linearized with Pme I and then transformed into Pichia pastoris GS115 by electroporation. The transformed cells were incubated in YPD liquid medium at 30°C for 2 hours, and then spread to YPDS agar plates (1% yeast extract, 2% peptone, 2% glucose, 1M sorbitol, 2% agarose, 1M sorbitol) containing 100, 700 or 1500 μg / mL Zeocin. After culturing at 30°C for 3 days, about 200, 4, and 0 single colonies grew on YPDS agar plates with 100, 700, or 1500 μg / mL Zeocin, respectively. Pick 5 (named L1~5) and 4 (named H1~4) single colonies on YPDS agar plates with 100 μg / mL Zeocin and 700 μg / mL Zeocin, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com