Method for producing somatic cell cloned bovine blastocyst
A technology of somatic cell cloning and donor cells, applied in biochemical equipment and methods, botany equipment and methods, applications, etc., can solve the problem of low efficiency of somatic cell cloning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
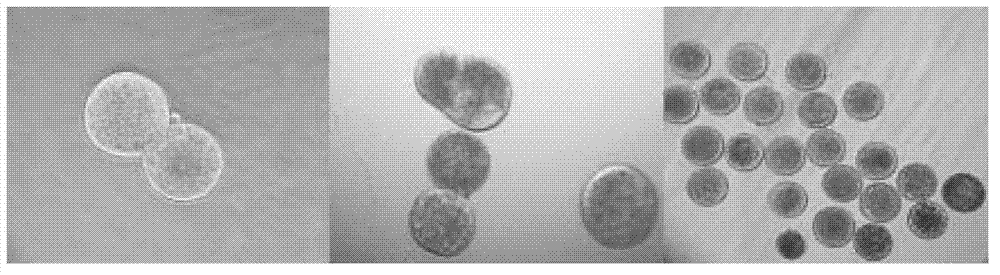
[0038] Example 1 Preparation of cloned bovine blastocysts by the somatic cell cloning method of the present invention
[0039] (1) Preparation of donor cells
[0040] Take 5-15 generations of Holstein bull ear margin fibroblasts, when the cells grow to 70-80% confluence, replace the DMEM medium containing 0.5% fetal bovine serum, continue to culture for 3-5 days, and then use them.
[0041] (2) Collection and in vitro maturation and culture of oocytes
[0042] Take cattle ovaries from the slaughterhouse, put them in normal saline at about 30℃, and transport them back to the laboratory in time. After washing the ovaries three times with normal saline, use a vacuum pump to suck follicles with a diameter of 2-8mm, and select the complete and dense cumulus-oocyte complexes (COCs) under a stereo microscope. First wash twice with 0.01% PVA-free calcium-magnesium PBS solution, then transfer it into TCM199+10%FBS+0.01IU / mL FSH+0.01IU / mLLH+1μg / mL E2 mature medium, 50-60 pieces / well. Place in...
Embodiment 2
[0050] Example 2 Preparation of cloned blastocysts by manual cloning
[0051] Among them, the preparation of donor cells, the collection and in vitro maturation and culture of oocytes, the activation and in vitro culture of reconstructed embryos are the same as the corresponding steps in Example 1. The oocytes are enucleated and zona-free oocytes are combined with the body. The cell fusion process is as follows:
[0052] Manually denuclear:
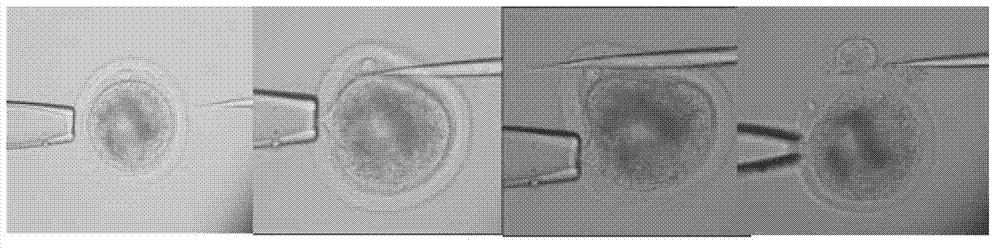
[0053] The oocyte-cumulus cell complex that has been matured in vitro for about 18 hours is treated with 0.1% hyaluronidase to remove granular cells, and then placed in a maturation solution with 5% decarboxycolcine (Demecolcine, DC) for 2 hours Then select the oocytes with uniform cytoplasm and discharge the polar body, digest the zona pellucida with 0.5% pronase, cut off the protruding part with a microdissecting knife under a stereo microscope, and put the non-nucleated oocytes into T20 Reserve in the droplet. see Figure 5 with 6 .
[0054...
Embodiment 3
[0057] Example 3 Preparation of cloned blastocysts by microinjection
[0058] Among them, the preparation of donor cells, the collection and in vitro maturation culture of oocytes, the activation and in vitro culture of reconstructed embryos are the same as the corresponding steps in Example 1. The construction process of oocyte denucleation and reconstructed embryos is as follows:
[0059] Microscopic denucleation:
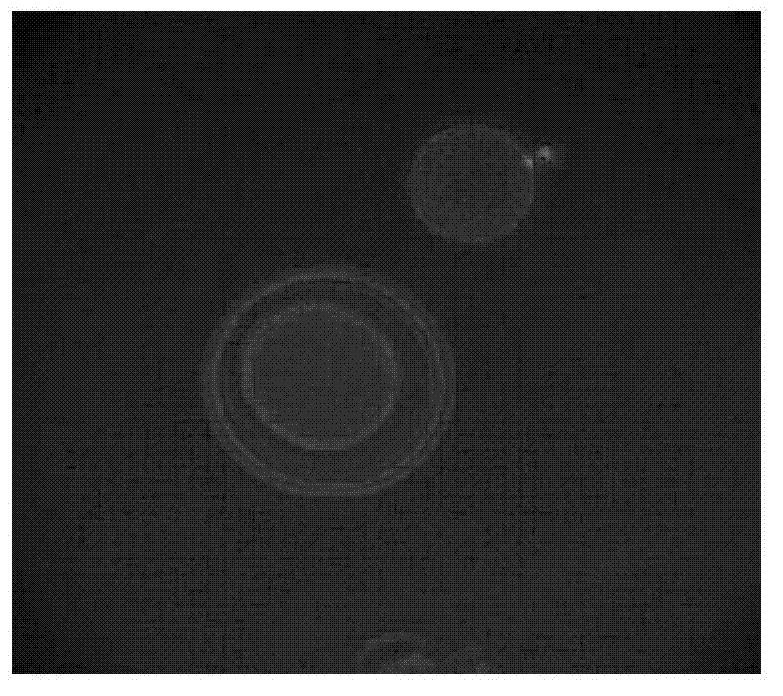
[0060] Move the oocytes with the first polar body into the operating solution H199+7.5ug / mL cell relaxin B droplet, and use a 20μm diameter glass pipette (with a tip) to remove the first polar body and The chromosomes in the oocyte below it are also sucked out. After the operation, the oocytes were transferred to the corresponding droplets in another dish, the cytoplasm containing the chromosomes was stained with Hoechst33342 for 5 minutes, and the enucleation rate was checked under UV light. The oocytes with complete enucleation were washed three times in T20 solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com