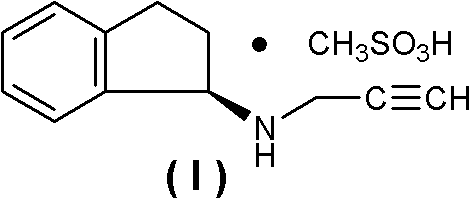
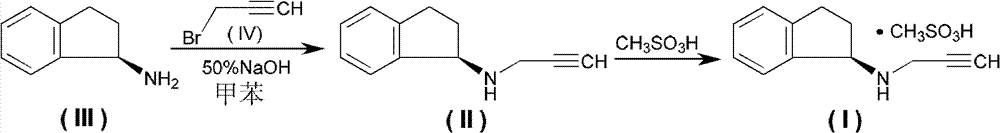
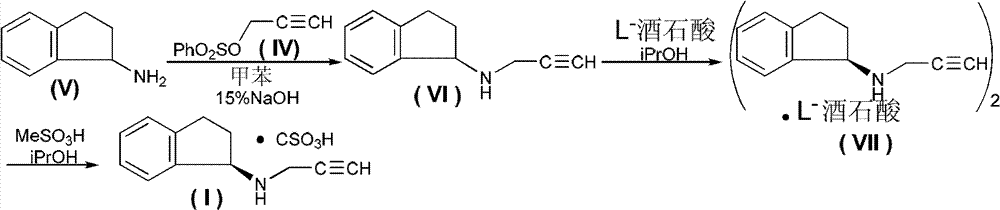
Method for preparing rasagiline mesylate
A technology of rasagiline mesylate and methanesulfonic acid, which is applied in the preparation of sulfonate, preparation of amino-substituted functional groups, organic chemistry, etc., can solve the health hazards of production operators and the working environment, and the additional cost of methanesulfonic acid , Increase the process and cost, etc., to save the salt-forming process and solvent consumption, reduce the production process and solvent usage, and simplify the production steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0023] Preparation example 1 propynyl alcohol methanesulfonate
[0024] Mix propynyl alcohol (2g, 35.7mmol), dichloromethane (20ml), and triethylamine (7.5ml, 53.7mmol), cool to 0°C, add methanesulfonyl chloride (3.3ml, 42mmol), after addition, the reaction solution Continue stirring at room temperature for 1 hour, quench the reaction with aqueous sodium bicarbonate, extract with dichloromethane (30ml×3), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter off the desiccant, and concentrate to obtain 4.2 g of propynyl alcohol methanesulfonate, yield: 88%. 1 HNMR (400MHz, CDCl 3 ): δ 4.85 (2H, d), 3.13 (3H, s), 2.73 (1H, t).
Embodiment 1
[0025] The preparation of embodiment 1 rasagiline mesylate
[0026] (R)-2,3-dihydro-1H-inden-1-amine (commercially purchased) (13.3g, 0.1mol), propynyl mesylate (26.83g, 0.2mol), acetonitrile (100ml) mixed , stirred and reacted at 45~50°C for 12 hours, stopped heating, naturally cooled to room temperature, filtered, the solid was washed with isopropanol (15ml×3), and dried to obtain 26.1g of white solid rasagiline mesylate, yield : 97.8%.
[0027] mp: 156-158°C; [α] D =+22.3° (c=2, EtOH);
[0028] 1 HNMR (400MHz, DMSO-d 6 ): δ9.50 (2H, br), 7.61 (1H, d), 7.42-7.29 (3H, m), 4.82 (1H, s), 4.02 (2H, s), 3.81 (1H, t), 3.17- 3.06 (1H, m), 2.94-2.84 (1H, m), 2.50-2.39 (1H, m), 2.32 (3H, s), 2.25-2.15 (1H, m).
Embodiment 2
[0029] The preparation of embodiment 2 rasagiline mesylate
[0030] (R)-2,3-dihydro-1H-indene-1-amine (35g, 0.26mol), propynol methanesulfonate (35.3g, 0.26mol), toluene (200ml) were mixed, and refluxed for 30 minutes , static, cooled to room temperature, a white precipitate precipitated, filtered by suction, and dried to obtain 64 g of white crystals, yield: 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com