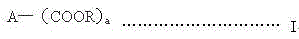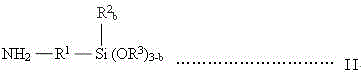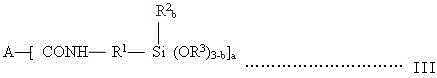A kind of synthesis technique of alkoxysilane containing amide group
The technology of aminoalkylalkoxysilane and alkoxysilane is applied in the field of synthesis technology of alkoxysilane, and can solve problems such as hydrolysis equipment and corrosion, and achieve the effects of improving reaction activity, avoiding corrosion, and being easy to separate and remove.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 500ml three-neck flask equipped with nitrogen conduit, tail gas liquid seal, stirrer, thermometer, reflux condenser and heating device, add 52.8g of dimethyl malonate, γ-aminopropylmethyldiethoxysilane 160.4g. Stir and heat to reflux at 90°C. After 7 hours of reaction, change the reflux device to a distillation device, and then heat at 90°C to distill the by-product methanol under normal pressure, and then distill the excess raw materials at 140°C under reduced pressure to obtain a light yellow liquid The product N,N'-bis[3-(diethoxymethylsilyl)propyl]malonamide 165.6g. The yield is 92%.
Embodiment 2
[0031] In a 1000ml four-neck flask equipped with a nitrogen conduit, tail gas liquid seal, dropping funnel, stirrer, thermometer, reflux condenser and heating device, add 194g of dimethyl terephthalate, γ-aminopropylmethyl Diethoxysilane 382g, sodium methoxide 1g. Stir and heat to reflux at 90-95°C, stop heating after 5 hours of reaction and cool the material to room temperature, start stirring again while adding 1.3g of dimethyldichlorosilane to neutralize the catalyst sodium methoxide through the dropping funnel, and then heat for 90 Atmospheric pressure distillation at ℃ to separate by-product methanol, dimethyldimethoxysilane, etc., and then distill off excess raw materials at 120 ℃ under reduced pressure. After cooling, the light yellow solid product N,N'-bis[3-( Diethoxymethylsilyl)propyl]terephthalamide 474g. Yield 92.6%.
Embodiment 3
[0033] Add 52.8 g of dimethyl malonate and 186 g of γ-aminopropyltriethoxysilane into a 500 ml three-neck flask equipped with a nitrogen conduit, tail gas liquid seal, stirrer, thermometer, reflux condenser and heating device. Stir and heat to reflux at 100°C. After 7 hours of reaction, change the reflux device to a distillation device, heat and distill the by-product methanol at 70°C under normal pressure, and then distill the excess raw materials at 130°C under reduced pressure to obtain a light yellow liquid product N,N'-bis[3-(triethoxysilyl)propyl]malonamide 181.6 g. Yield 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com