Preparation method of controlled release antibacterial film
A technology of lysozyme and film-forming matrix is applied in the field of preparation of controlled-release antibacterial films, which can solve the problems of slow release rate and fast release rate, and achieve the effects of reducing damage, improving efficiency, and increasing the upper limit of addition amount.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Accurately weigh 0.5g of zein and 0.1g of thymol, dissolve in 20mL of 80% ethanol-water solution; dissolve 0.5g of sodium caseinate in 50ml of deionized water, quickly pour it into the stirring alcohol solution, At a temperature of 50 ° C, a vacuum of 0.1 Mpa was rotated for 20 min, centrifuged at 8000 rpm for 5 min and freeze-dried. Dissolve 1g of gelatin in 20ml of water, stir at 60°C for 10min, add 0.2g of freeze-dried nanoparticle powder, 40mg of lysozyme and 0.24g of glycerin, pour the film after stirring, and dry at 40% RH and 25°C for 24 hours. A mixed film is obtained.
[0041] The physicochemical performance parameter of the nanoparticle powder that embodiment obtains is as follows:
[0042] The particle size is 182.8nm, the PDI value is 0.22, the potential is -39.2mv, the yield is 79.4%, and the encapsulation efficiency is 84.6%.
[0043] The nano particle powder of the invention can be used as a food protein nano antibacterial embedding and delivery carrier...
Embodiment 2
[0047] Accurately weigh 0.5g of zein and 0.1g of thymol, dissolve in 20mL of 70% ethanol-water solution; dissolve 0.5g of sodium caseinate in 50ml of deionized water, quickly pour it into the stirring alcoholic solution, At a temperature of 60 ° C, a vacuum of 0.1 Mpa was rotated for 10 min, centrifuged at 1000 rpm for 10 min and freeze-dried. Dissolve 1g of sodium caseinate in 20ml of water, add 0.2g of freeze-dried nanoparticle powder, 40mg of lysozyme and 40u of TG enzyme, stir at 45°C for 4 hours, add 0.48g of glycerin, pour the membrane after stirring, and put it in RH40 %, and dried at 25°C for 24 hours to obtain a cross-linked mixed film.
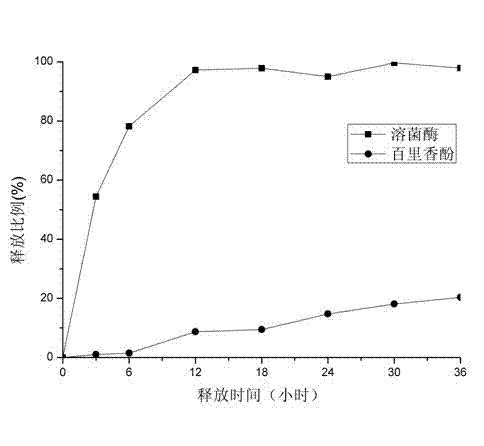
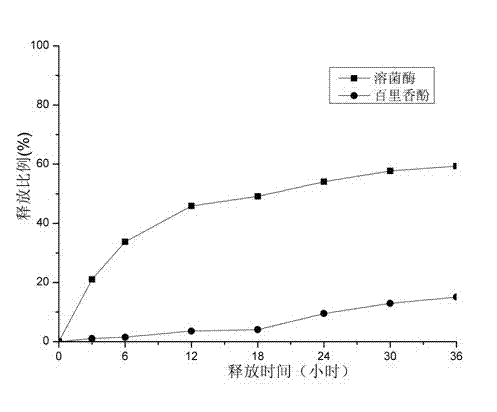
[0048] The result of its release is as figure 2 shown.
[0049] From figure 2 It can be seen that the release rate of directly loaded lysozyme is still faster, and the release rate of loaded thymol after embedding is still slow. However, the rate of release has been greatly reduced compared to that of uncrosslinked membranes. ...
Embodiment 3
[0053]Accurately weigh 0.5g zein, dissolve it in 20mL 90% ethanol-water solution; , rotary evaporated at a vacuum of 0.1 Mpa for 20 min, centrifuged at 8000 rpm for 5 min and freeze-dried. Take 0.2 g of freeze-dried nanoparticle powder and 1 g of sodium caseinate, dissolve them in 30 ml of water, add 0.36 g of glycerin, pour the film after stirring, dry at RH40%, 25 ° C for 24 hours to obtain the sample film.
[0054] The physical and chemical performance parameters of nanoparticle powder are as follows:
[0055] The particle size is 184.2nm, the PDI is 0.22, the potential is -37.2mv and the yield is 79.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com