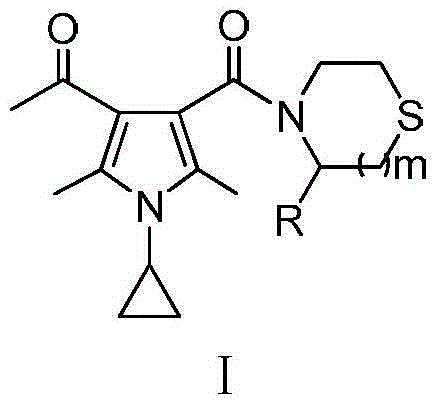
Thiomorpholine-containing pyrrole derivatives and their preparation method and use
A technology of drugs and compounds, applied in the field of medicine, can solve problems such as weak alkalinity, limitation, and difficulty in purification, and achieve the effect of obvious platelet aggregation and inhibition of platelet aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
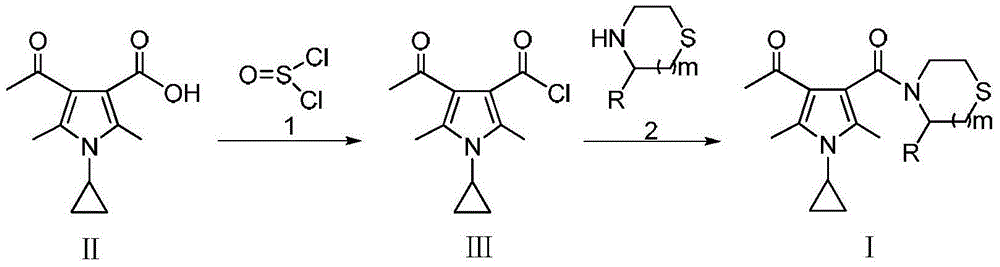
[0057] Preparation of Intermediate Ⅲ-1
[0058]
[0059] Add 22.1g (0.01mol) 4-acetyl-1-cyclopropane-2,5-dimethyl-1H-pyrrole-3-carboxylic acid (II) into a reaction flask equipped with a stirrer, a condenser and a thermometer. Dissolve it with 100ml of thionyl chloride, stir, and react under reflux for 4h (the plate layer shows that the reaction is complete). The thionyl chloride was evaporated to dryness to obtain a white solid (HPLC: 99.6%). HRMS(m / z)[M+H] + : 240.0793.
Embodiment 1
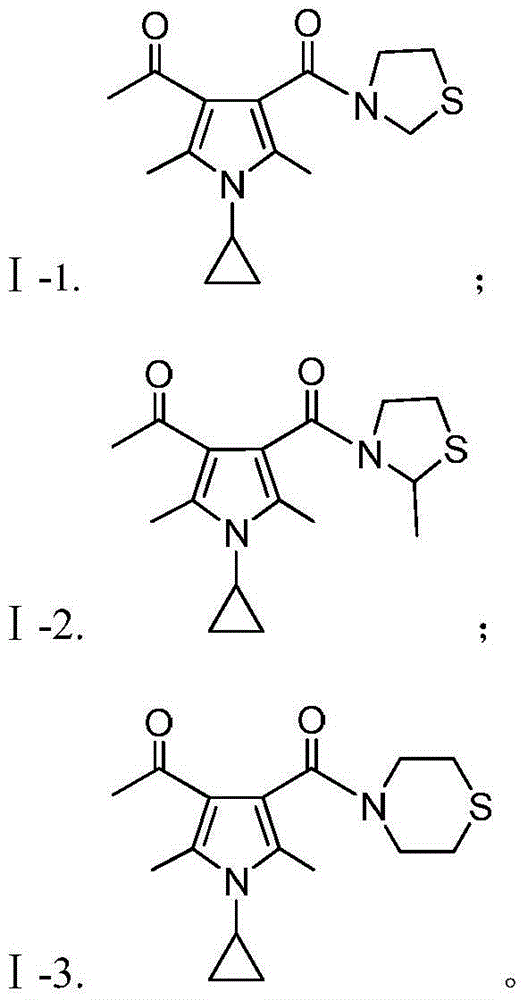
[0061] Preparation of 1-(1-cyclopropyl-2,5-dimethyl-4-(thiazolidine-3-carbonyl)-1H-pyrrol-3-yl)ethanone (Compound Ⅰ-1)
[0062]
[0063] Add 24.0g (0.01mol) of Intermediate III-1 into a reaction flask equipped with a stirrer, condenser and thermometer, dissolve it with 150ml of dichloromethane, and add 20.2g (0.2mol) of triethylamine with stirring. 8.9 g of thiazolidine was added to the reaction system. After adding, continue to react for 5h under reflux (the plate layer shows that the reaction is complete). The reaction solution was washed with 3×50ml water, the methylene chloride layer was fully dried with anhydrous sodium sulfate, filtered, and the methylene chloride was evaporated under reduced pressure to obtain a pale yellow solid, which was separated by column [mobile phase: v (petroleum ether): v (Ethyl acetate)=1:2], a white solid (HPLC: 99.7%) can be obtained. HRMS(m / z)[M+H] + : 293.1279.
[0064] Referring to Example 1, compounds I-2 to I-3 can be synthesized.
[0065]...
Embodiment 2
[0068] Compound I-1 into hydrochloride: Take 2.9 g of I-1 white solid and dissolve it in 15 mL of absolute ethanol. Cool to 5°C in an ice-water bath, add 11.1% hydrochloric acid ethanol solution dropwise to pH 2, and continue to stir in an ice-water bath for about 1 hour. After filtration, a light yellow solid was obtained, which was dried in vacuum, m.p.>230°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com