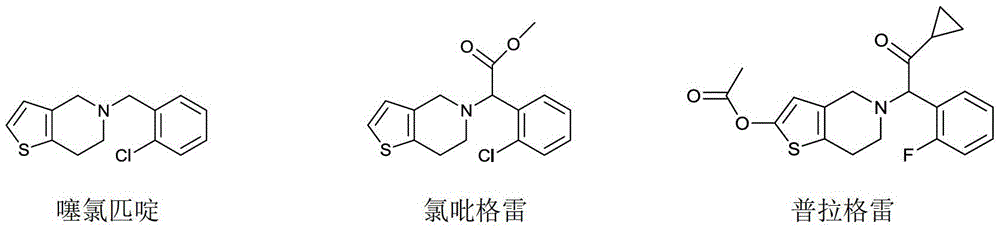
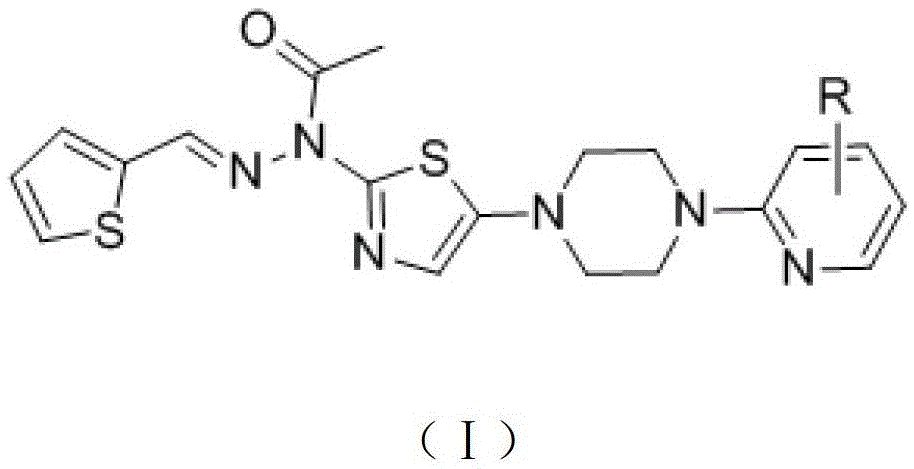
A class of compounds with antithrombotic effect
A compound and pharmaceutical technology, applied in the field of medicine, can solve the problems of high bleeding risk and excessive bleeding in patients, and achieve the effect of inhibiting platelet aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Compound Ⅳ-1
[0049]
[0050] Take compound Ⅱ-1 (10g, 61mmol) and dissolve it in 50mL of dichloromethane. Stir well to dissolve. Cool down to 0°C. A dichloromethane solution of bromoacetyl bromide (III, 13.6 g, 67 mmol) was added dropwise. After dropping, keep the temperature and react for 4h. After the reaction was detected by TLC, the reaction solution was washed three times with water (30mL×3), the organic layer was separated, dried, filtered, and rotary evaporated. Wait until the light yellow oil, without refining, can be used for the next reaction. The quality of the obtained product is 16.5g, the yield is 95%, the purity is 98.3% (HPLC normalization method), MS (m / z) [M+H] + : 284.0.
[0051] With reference to Example 1, the following compounds can be obtained
[0052]
Embodiment 2
[0054] Compound Ⅵ-1
[0055]
[0056] Dissolve compound IV-1 (10g, 35mmol) in 50mL of ethanol, add potassium acetate (10.3g, 105mmol) after fully dissolved, heat to reflux, react for 6h, TLC detects that the reaction is complete. The reaction solution was left to cool, poured into water, and a solid precipitated out. Filter, dry, and recrystallize with ethyl acetate and petroleum ether 1:2 to obtain 10.5g of product, yield 80.8%, purity 99.1% (HPLC normalization method), MS(m / z)[M+H] + : 371.1.
[0057] With reference to Example 2, the following compound can be obtained
[0058]
Embodiment 3
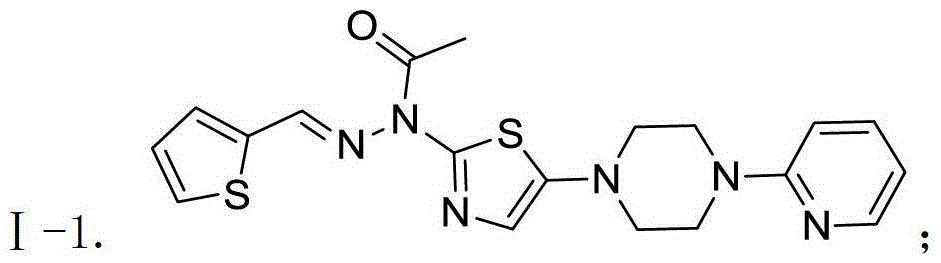
[0060] Compound Ⅰ-1
[0061]
[0062] Take compound IV-1 (5g, 13.5mmol) and fully dissolve it with 30mL of dichloromethane. Decrease to 0°C, add triethylamine (2g, 20mmol), dropwise add acetic anhydride (2.1g, 20mmol), keep the temperature for 3h. TLC detects that the reaction is complete. The reaction solution was poured into 30 mL of water, the organic phase was separated and washed three times with water (30 mL×3). The organic layer was dried, filtered and evaporated to dryness to give a brown oil. Stir with ethanol at room temperature to obtain a white solid, which is filtered, and the filter cake is rinsed with cold ethanol to obtain 4.3 g of the product. Yield 77.2%, purity 99.4% (HPLC normalization method), MS(m / z)[M+H] + : 413.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com