Antituberculosis compositions of byttneria species
The technology of a kind of Echinopsis genus, composition, is applied in the field of the composition comprising the extract of Echinopsis genus species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Protocol for the identification of MtbGS inhibitors:
[0045] The test mixture contained 50 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), pH 6.8 buffer, 220 mM L-glutamic acid (pH adjusted to 6.8), 7.5 mM chloride Ammonium, 32.5 mM magnesium chloride and 62.5 μg / ml enzyme (Mycobacterium tuberculosis glutamine synthetase). To 85 μl reaction mixture was added 5 μl of the sample (Pipa japonica extract). 5 μl DMSO (dimethyl sulfoxide) was added to the control and 5 μl 420 mM EDTA (ethylenediaminetetraacetic acid) was added to the blank. Reactions were initiated by adding 7.6 mM ATP (pH adjusted to ~6.8) to the reaction mixture and incubated at 25°C for 2 hours. The reaction was terminated by adding a stopping reagent followed by citrate to block further hydrolysis of ATP. The whole mixture was left at room temperature for an additional 30 minutes to read the color by a SpectraMax plate reader at 655 nm. Methanol extracts of Echinopsis sp. wer...
Embodiment 2
[0047] Example 2: Column Chromatography of the Methanol Extract of Example 1:
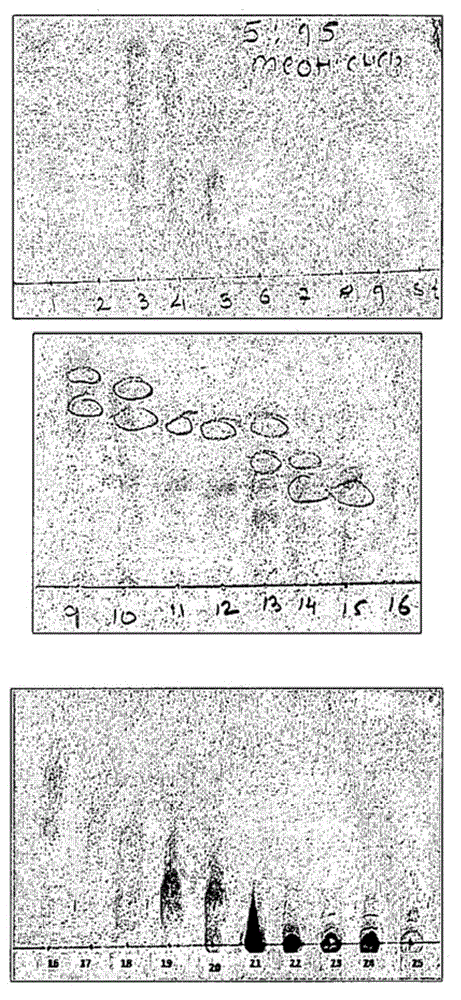
[0048] Chromatography was performed on a sample of 19 gms through 200 gms of silica gel (100-200 mesh) using a gradient mobile phase according to Table-1. 25 fractions were collected at this stage. Use 5:95MeOH:CHCl 3 Developed phase TLC of all these collected fractions was performed on alumina-coated silica gel TLC plates to check purity. Fractions showing similar TLC (Panel-I) patterns were pooled and placed in 15 ml glass test tubes to obtain 11 fractions (A, B, C, D, E, F, G, H, I , J, K). After removal of the solvent, the bioactivity of these fractions was evaluated using the protocol mentioned in Example 1. Fractions D (fraction 4) and K (fraction 11) were found to exhibit activity against MtbGS.
[0049] Table I
[0050]
Embodiment 3
[0051] Example 3: Purification of Fraction D (Fraction 4):
[0052] Re-chromatography was performed on 100 mg of Fraction D using 36 gms of silica gel (100-200 mesh) and mobile phase as described in Table II. Perform TLC of all collected fractions (Figure-2). After analysis of the TLC plate, the fractions were pooled into 10 final fractions D1-D10. An assay as in Example 1 was carried out to identify the active fraction. Fraction D4 was found to be active.
[0053] Table-II
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com