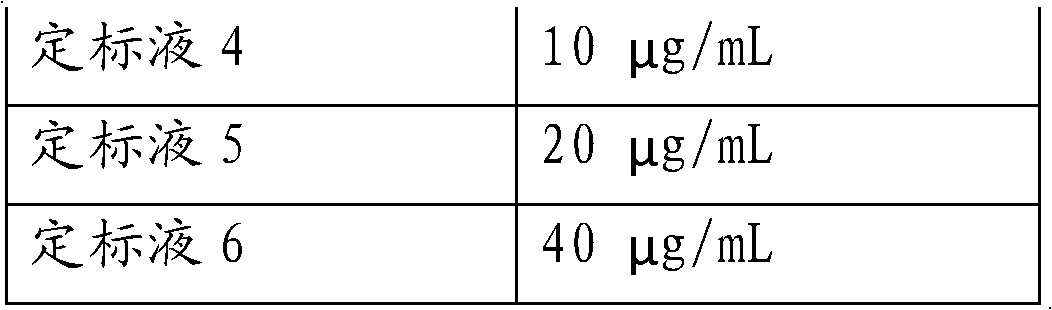Preparation method of phenytoin homogeneous enzyme immunoassay kit and phenytoin polyclonal antibodies
A polyclonal antibody and detection kit technology, applied in the field of medical testing, can solve the problems of complex operation, high reagent cost, high detection cost, etc., and achieve the effects of strong specificity, high accuracy and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of phenytoin-specific polyclonal antibody:
[0036] a. Synthesis of phenytoin immune antigen
[0037] 1) Activation of phenytoin derivatives. Weigh 20 mg of specific phenytoin derivatives into a small beaker, and add 700 μL of pure dimethylformamide (DMF), 700 μL of absolute ethanol, and 1.4 mL of 10 mM pH Potassium phosphate buffer solution of 5.0, 80mg1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide and 10mgN-hydroxyl sulfosuccinimide (N-hydroxysuccinimide, Sulfo-NHS), will These chemicals were stirred and dissolved for 30 minutes at room temperature;
[0038] 2) Preparation of BSA solution, weighing 40mg of bovine serum albumin (Bovine Serum Albumin, BSA) and dissolving it in 10mL of 0.2M pH 8.5 phosphate buffer at room temperature;
[0039] 3) Coupling and purification of the antigen, the activated phenytoin derivative is added dropwise to the BSA solution, stirred overnight at 2-8°C to obtain the antigen; and the coupled antigen is dialyzed and purifie...
Embodiment 2
[0044] The preparation of phenytoin homogeneous enzyme immunoassay kit includes the following steps: preparation of phenytoin derivative-specific polyclonal antibody, preparation of glucose hexaphosphate dehydrogenase-labeled phenytoin conjugate, preparation of calibrator Preparation, preparation of homogeneous enzyme immunoassay reagents and sample testing using an automatic biochemical analyzer
[0045] a. Prepare phenytoin-specific polyclonal antibody according to the method of Example 1.
[0046] b. Preparation of phenytoin enzyme-labeled antigen
[0047] 1) Weigh 30mg of glucose hexaphosphate dehydrogenase, dissolve it in 24mL, 0.05M Tris buffer, then add 200-500mg of NADH, 1.0-2.0mL of carbitol and 2-6.0mL of dimethyl methylene Sulfone mix;
[0048] 2) Dissolve 20 mg of phenytoin derivative in 840 μL dimethyl sulfoxide and 360 μL DMF, add 12 μL tributylamine and 6 μL isobutylchloroformate, and stir at 2-8 °C 30min;
[0049] 3) The above two solutions were mixed, stir...
Embodiment 3
[0069] Inspection experiment of phenytoin test kit products
[0070] a. Recovery experiment
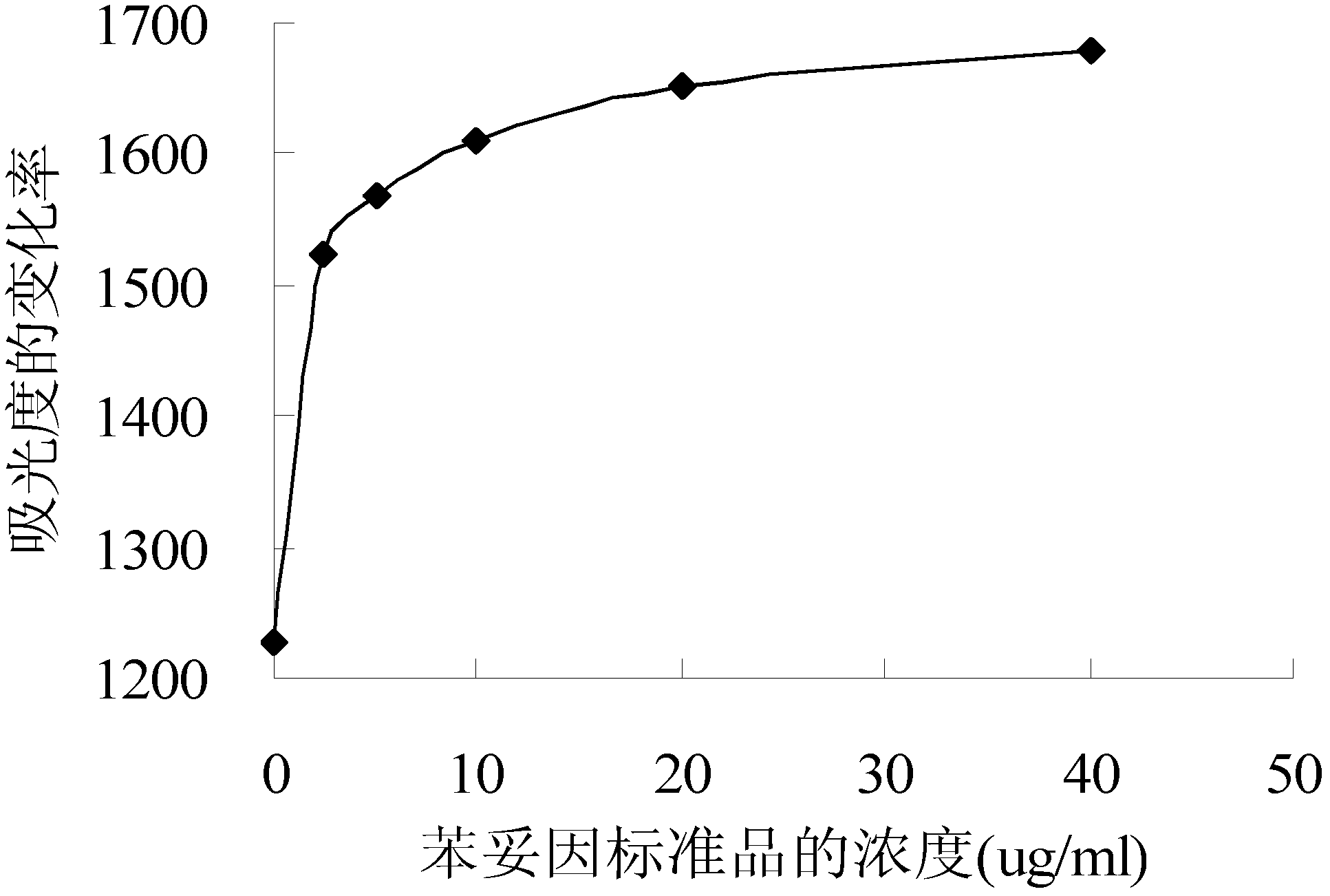
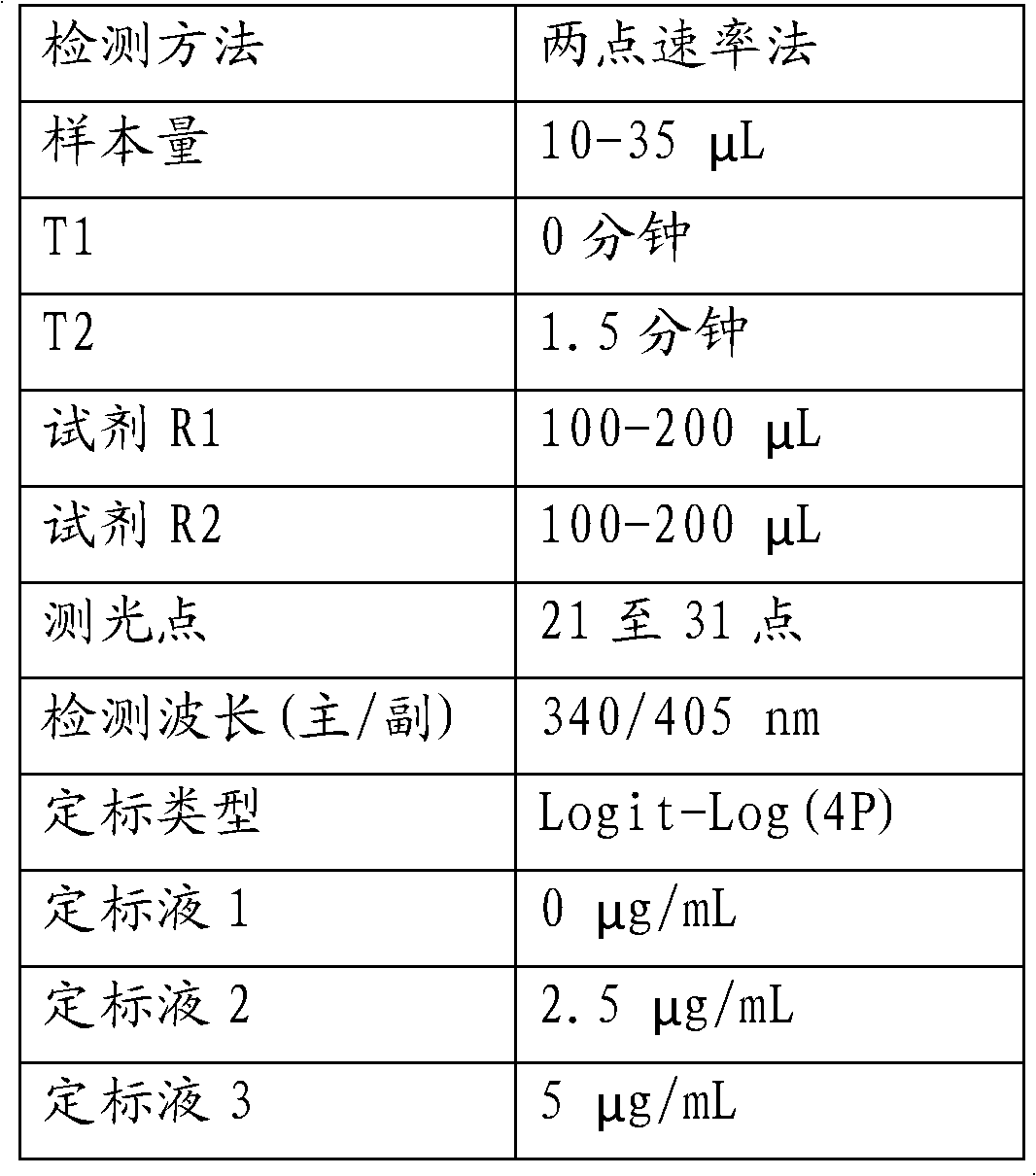
[0071] Using the established phenytoin calibration curve, determine three concentrations of phenytoin serum samples: low (2.5 μg / mL), medium (10 μg / mL) and high (40 μg / mL) concentrations, and repeat the measurement 5 times for each sample .
[0072] Table 2: Recovery experiment of phenytoin kit
[0073]
[0074] The specific results are shown in Table 2. The average recovery rate (Recovery) of the sample test is greater than 95%, the standard deviation (SD) is less than 1.44, and the correlation coefficient deviation (CV) is less than 8%.
[0075] b. Drug interference experiment
[0076] Select 32 commonly used compounds and drugs, adjust their working concentration to 10.0 μg / mL, and conduct interference test on Hitachi 7180 automatic biochemical analyzer. The test results are shown in Table 3:
[0077] Table 3: Drug Interference Experiment of Phenytoin Kit
[0078]
[007...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com