High-purity epoxy compound and method for producing same
A technology of epoxy compound and manufacturing method, which is applied in the direction of organic chemical methods, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of unpurified distillation, high viscosity, low purity, etc., and achieve less impurity content and lower Effect of high viscosity and chemical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
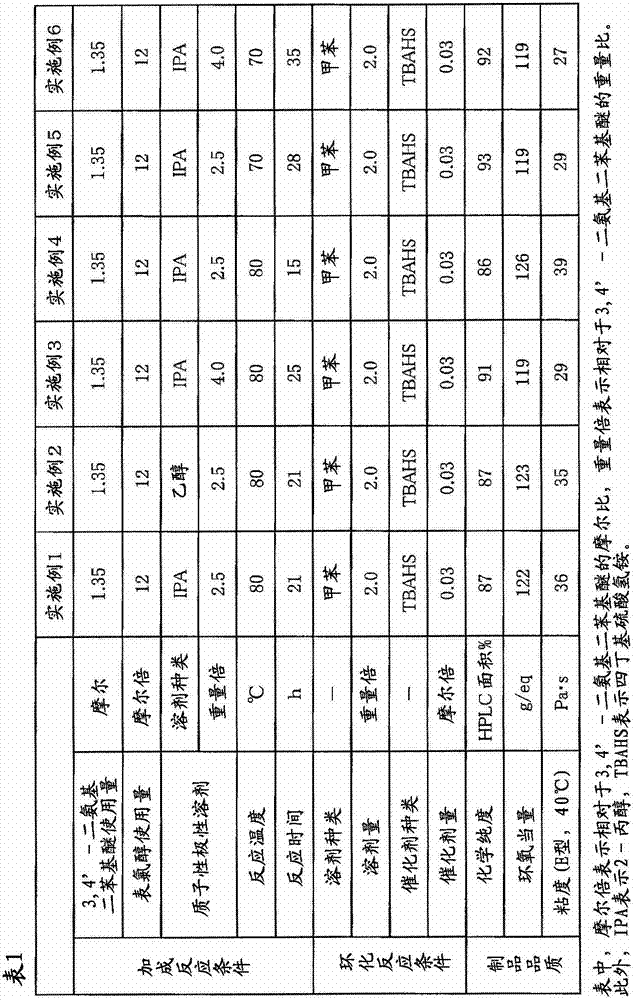
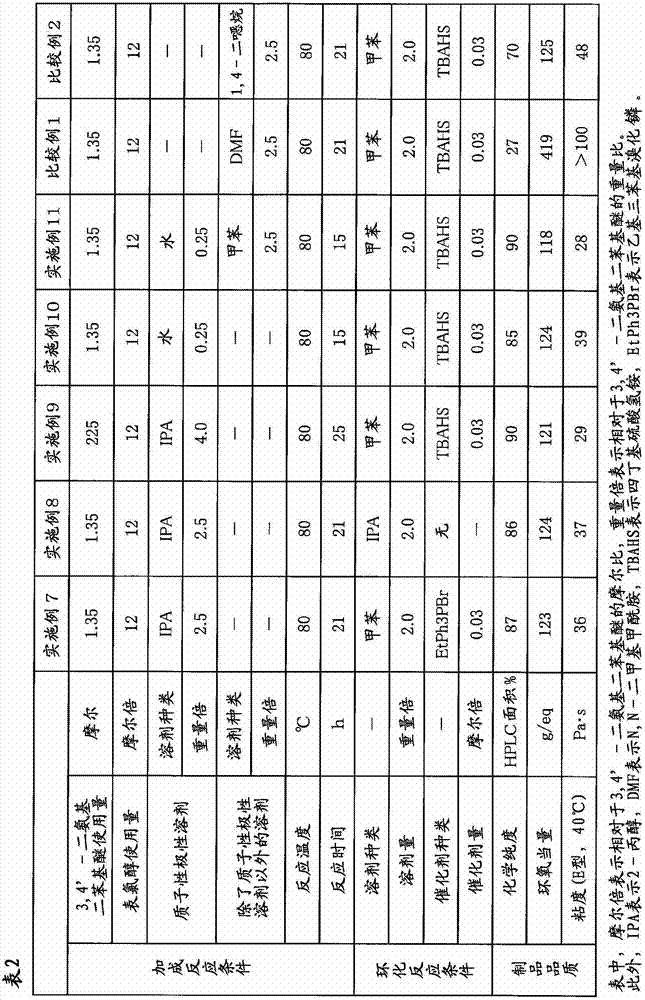
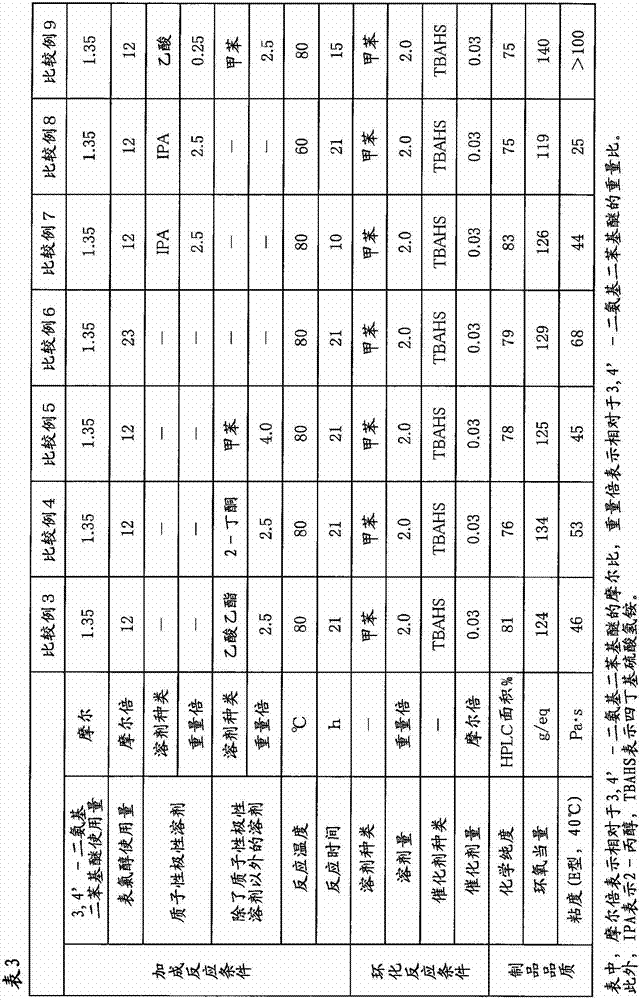
[0090] In a four-necked flask equipped with a thermometer, a cooling tube, and a stirrer, add 1500 g (16.2 moles) of epichlorohydrin and 675 g (2.5 times by weight / 3,4'-diaminodiphenyl ether) of 2-propanol. 270 g (1.35 mol) of 3,4'- diamino diphenyl ether was added. This mixed solution was stirred at a temperature of 80° C. for 21 hours to perform an addition reaction. From the addition reaction liquid, 1178 g of 2-propanol and a part of remaining epichlorohydrin were distilled off under reduced pressure. To the obtained concentrate, 540 g of toluene (2.0 times by weight / 3,4'-diaminodiphenyl ether) and 13.8 g (0.041 mol) of tetrabutylammonium bisulfate as a catalyst for the cyclization reaction were added, and then 675 g (8.1 mol) of 48% sodium hydroxide was added dropwise over 30 minutes at a temperature of °C, and then aged while stirring at a temperature of 30 °C for 4 hours to perform a cyclization reaction. After completion of the cyclization reaction, the generated sal...
Embodiment 2
[0092] In Example 1, the same procedure as in Example 1 was carried out except that 2-propanol was changed to 675 g of ethanol (2.5 times by weight / 3,4'-diaminodiphenyl ether). 575 g of a brown viscous liquid containing N,N,N',N'-tetraglycidyl-3,4'-diaminodiphenyl ether as a main component (100% of theoretical yield) was obtained. The chemical purity of this epoxy compound was measured by the method described above using HPLC, and it was 87% (HPLC area %). The epoxy equivalent was 123 g / eq, and the viscosity measured at 40° C. using an E-type viscometer was 35 Pa·s.
Embodiment 3
[0094] In Example 1, the amount of 2-propanol as an addition reaction solvent was changed from 675 g to 1080 g (4.0 weight times / 3,4'-diaminodiphenyl ether), and the reaction time of the addition reaction was changed from It implemented similarly to Example 1 except having changed 21 hours into 25 hours, and having changed the quantity of the 2-propanol distilled off from the addition reaction liquid, and the residual epichlorohydrin into 1620 g. 565 g of a brown viscous liquid containing N,N,N',N'-tetraglycidyl-3,4'-diaminodiphenyl ether as a main component (99% of theoretical yield) was obtained. The chemical purity of the epoxy compound was measured by the method described above using HPLC, and it was 91% (HPLC area %). The epoxy equivalent was 119 g / eq, and the viscosity measured at 40° C. using an E-type viscometer was 29 Pa·s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Epoxy equivalent | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com