Method for synthesizing 3-hydroxy-azetidinehydrochloride
A technique for the synthesis of azetidine, which is applied in the field of compound preparation, and can solve the problems of expensive benzhydrylamine, high energy consumption, and long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthetic method of 3-hydroxyl-azetidine hydrochloride, comprises the following steps:
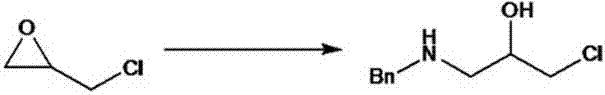
[0030] (1) Dissolve benzylamine in 15 times the mass of water, cool down to 0-5°C, slowly add epichlorohydrin (1.3 equivalents) to the above reaction solution, maintain the system at 0-5°C, and react for 12 hours. GC monitoring raw material reaction is complete. Filter, wash the filter cake twice with 2 times the mass of water, and then wash once with an organic solvent with a volume ratio of 2 times the mass of ethyl acetate:petroleum ether=1:20. Let it dry to get the product of the first step. HPLC detection, purity greater than 96%, used directly for the next step. Yield 89%.
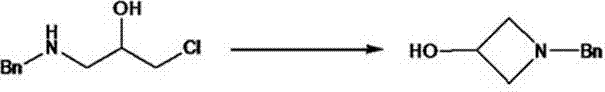
[0031] (2) Dissolve the compound prepared in the first step in 15 times the mass of acetonitrile, add 1.5 equivalents of sodium carbonate, and heat the reaction system to reflux. The reaction was carried out for 12 hours, and the reaction of the raw materials was detected by HPLC. After cooling an...
Embodiment 2
[0034] The synthetic method of 3-hydroxyl-azetidine hydrochloride, comprises the following steps:
[0035] (1) Dissolve 1 kg of benzylamine in 15 kg of water, cool down to 0°C, slowly add 1.117 kg of epichlorohydrin to the above reaction solution, keep the system not exceeding 5°C during the whole addition process, stir and maintain At this temperature, react for 12 hours, HPLC detects that benzylamine has reacted completely, return to room temperature, filter the reaction solution, wash the filter cake twice with 2 kg of water, and then use 2 kg of ethyl acetate:petroleum ether=1 : 20% organic solvent washing, product dries, obtains 1649 grams of product, HPLC detects purity 96.7%, yield 88.7%
[0036] (2) Dissolve 1649 grams of the compound obtained in the first step in 25 kilograms of acetonitrile, add 878 grams of sodium carbonate, then heat to reflux, react for 12 hours, and detect by HPLC, the reaction of the raw materials is complete,
[0037] Cooled to room temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com