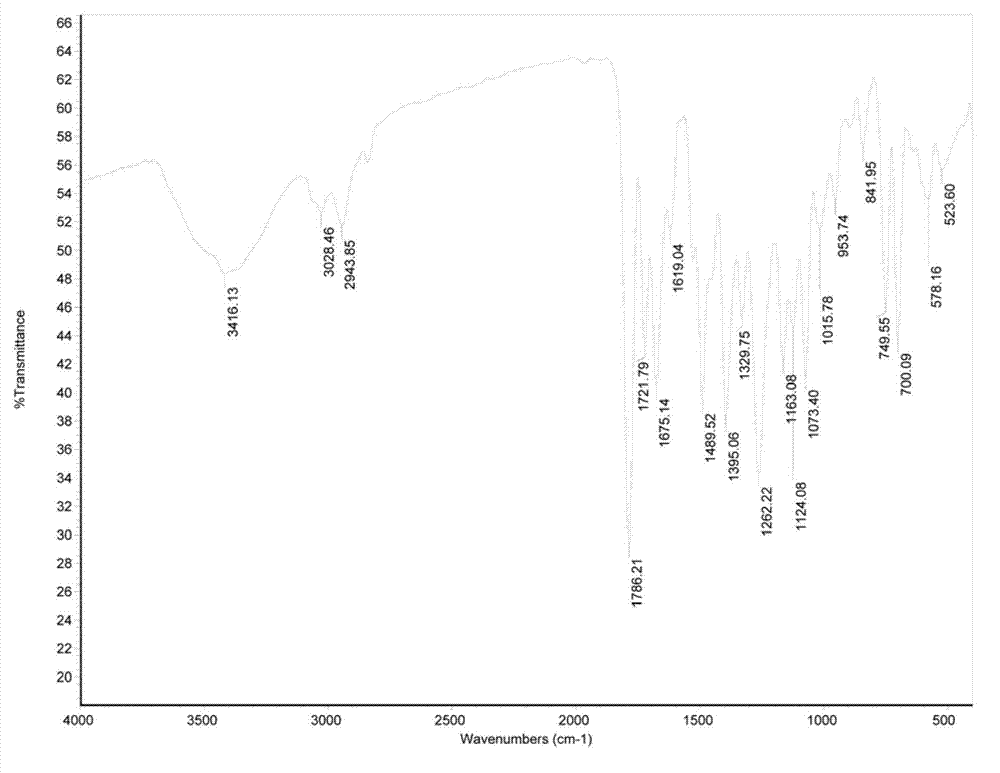
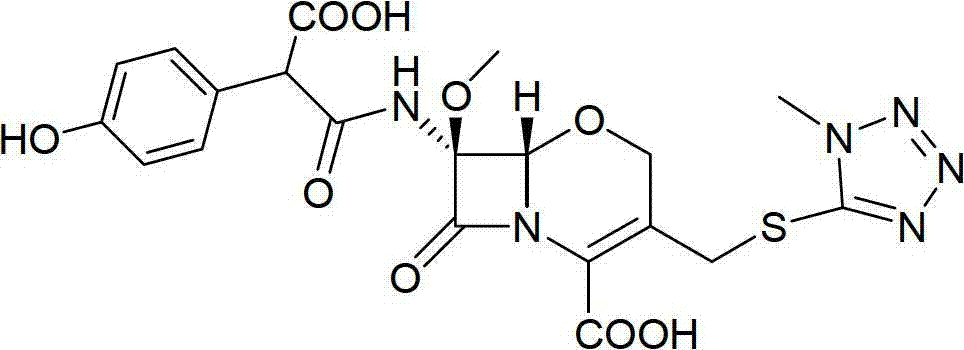
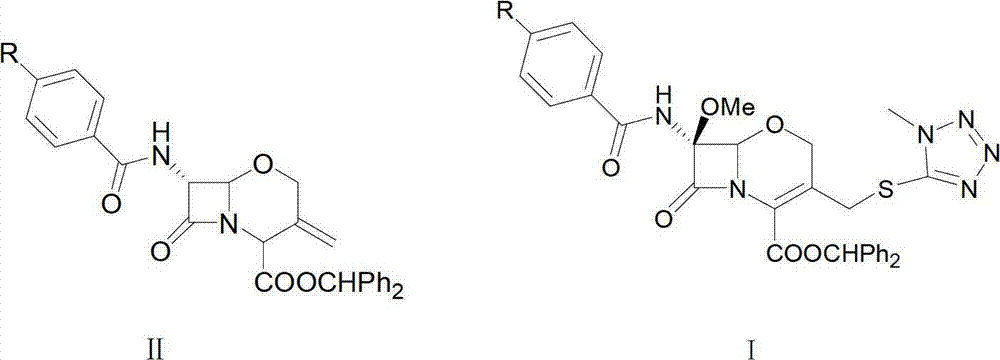
Method for preparing oxygen cephalosporin compound
A compound, oxycephalosporin technology, applied in the field of pharmaceutical synthesis, can solve the problems of high production equipment requirements, unfavorable industrial production, long reaction steps, etc., and achieves the effects of being beneficial to production operations, beneficial to industrial production, and short reaction routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Formula I compound 7-(benzoyl)amino)-7-methoxy-(3-((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5 -The preparation method of oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid diphenylmethyl ester:
[0041]
[0042]Add 500mL of dichloromethane into a 2000mL four-neck flask, then add 47g (0.1mol) of the compound of formula II, wherein R in the compound of formula II is hydrogen, stir until dissolved and clear, cool down to -45°C with liquid nitrogen, and then slowly add 15g (0.14mol) of tert-butyl hypochlorite, start dropwise adding methanol solution of lithium methoxide, of which 15g (0.4mol) of lithium methoxide and 150mL of methanol, control the temperature at -45°C during the dropwise addition, after the dropwise addition , continue to react under the condition of controlling the temperature at -45°C for 2.0 hours. After the reaction, use TLC to detect, the mobile phase is petroleum ether: ethyl acetate ratio of 2:1, the test result shows that the reaction is com...
Embodiment 2
[0045] Formula I compound 7-(benzoyl)amino)-7-methoxy-(3-((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5 -The preparation method of oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid diphenylmethyl ester
[0046]
[0047] Add 550mL of chloroform into a 2000mL four-neck flask, then add 47g (0.1mol) of the compound of formula II, wherein R in the compound of formula II is hydrogen, stir until dissolved and clear, cool down to -50°C with liquid nitrogen, and then slowly add 10.8g (0.1mol) tert-butyl hypochlorite, start dropwise adding the methanol solution of lithium methoxide, wherein, the lithium methoxide in the methanol solution of lithium methoxide is 19g (0.5mol), the methanol is 200mL, and the temperature is controlled at -50 during the dropwise addition. ℃, after the dropwise addition is completed, continue to react under the condition of controlling the temperature at -50°C for 2.5 hours. Add 20mL of acetic acid, stir for 20 minutes, then add 500mL of sodium thiosul...
Embodiment 3
[0050] Formula I compound 7-(benzoyl)amino)-7-methoxy-(3-((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5 -The preparation method of oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid diphenylmethyl ester
[0051]
[0052] Add 550mL of dichloromethane and 50ml of dioxane into a 2000mL four-neck flask, then add 47g (0.1mol) of the compound of formula II, wherein R in the compound of formula II is hydrogen, stir until dissolved and clear, and cool down to -60°C, then slowly add 21.7g (0.2mol) of tert-butyl hypochlorite, and start to add the methanol solution of lithium methoxide dropwise. Among them, the amount of lithium methoxide in the methanol solution of lithium methoxide is 13.3g (0.35mol), and the amount of methanol is 180mL , during the dropwise addition, the temperature was controlled at -60°C. After the dropwise addition, the reaction was continued at -60°C for 3.0 hours. After the reaction, TLC was used for detection, and the mobile phase was petroleum ether: ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com