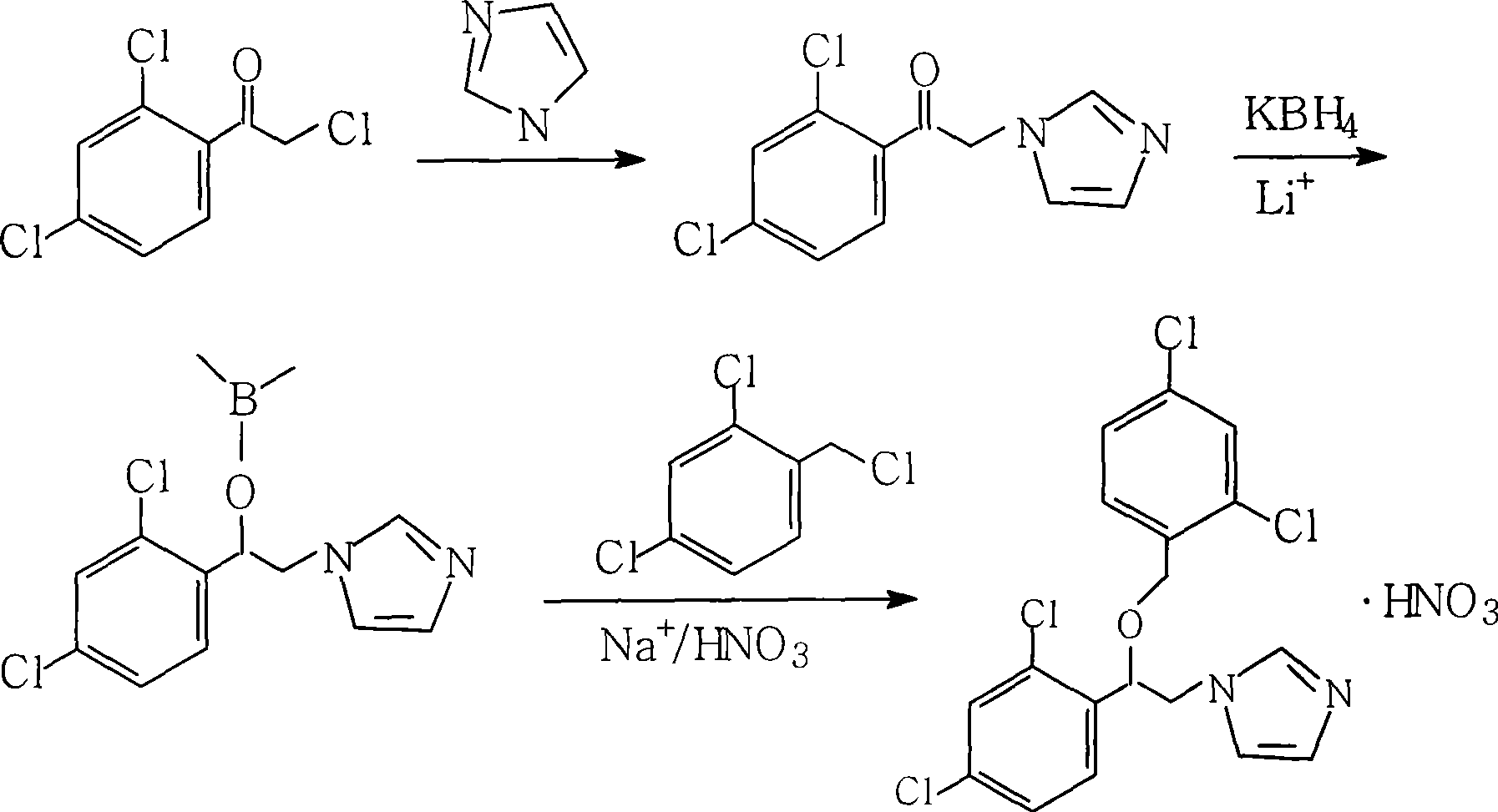
Method for industrial production of miconazole nitrate
A technology of miconazole nitrate and imidazole, applied in the directions of organic chemistry, antifungal agent, etc., can solve the problems of difficult N-alkylation reaction of imidazole, affecting reaction yield, reducing chemical activity, etc., so as to avoid the influence of side reactions, The effect of utilizing the reactivity and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Put 8g of imidazole, 18mL of triethylamine, and 150ml of benzene in a 500mL three-neck flask, heat to 70°C and stir for 20min, and slowly add the benzene solution of 2,4-dichloro-α-chloroacetophenone dropwise after the raw materials are completely dissolved (Containing 22.35g of 2,4-dichloro-α-chloroacetophenone), react at 70°C for 1.5h. After the reaction is completed, filter through a filter cloth to remove triethylamine hydrochloride, pour the reaction solution into 1% hydrochloric acid water with a concentration three times its volume for full washing, and fully remove triethylamine hydrochloride and unreacted imidazole. Use a separatory funnel to separate the water layer, dry the organic layer by adding anhydrous calcium chloride, add the dried reaction solution into a three-necked reaction flask, add 2.1 g of phase transfer catalyst PEG600, 3.8 g of potassium borohydride, and 2.3 g of lithium carbonate. Reduction at 70°C for 5h. After the reaction was completed, ...
Embodiment 2
[0028]Put 8g of imidazole, 18mL of triethylamine, and 150ml of benzene in a 500mL three-neck flask, heat to 40°C and stir for 20min, and slowly add the benzene solution of 2,4-dichloro-α-chloroacetophenone dropwise after the raw materials are completely dissolved (containing 22.35 g of 2,4-dichloro-α-chloroacetophenone), reacted at 40° C. for 3.0 h, poured the reaction solution into water containing 1.5% hydrochloric acid three times its volume, and washed fully three times. Use a separatory funnel to separate the water layer, add anhydrous calcium chloride to the organic layer to dry, add the dried reaction solution into a three-necked reaction flask, add 2.1 g of phase transfer catalyst PEG400, 3.8 g of potassium borohydride, and 2.0 g of lithium chloride. Reduction at 40°C for 10h. After the reaction was completed, it was cooled to 28°C, 15g NaOH and 4ml of water were directly added to the reaction liquid, and then 16.3g of 2,4-dichlorobenzyl chloride was added dropwise, an...
Embodiment 3
[0030] Put 8g of imidazole, 18mL of triethylamine, and 150ml of toluene in a 500mL three-neck flask, heat to 60°C and stir for 30min, and slowly add the benzene solution of 2,4-dichloro-α-chloroacetophenone dropwise after the raw materials are completely dissolved (Containing 22.35g of 2,4-dichloro-α-chloroacetophenone), react at 60°C for 2.0h. After the reaction is completed, filter through filter paper, pour the reaction solution into water containing 1% hydrochloric acid with a concentration three times its volume, and wash it three times, use a separatory funnel to separate the water layer, add anhydrous calcium chloride to the organic layer, and dry it. The reaction solution was put into a three-necked reaction flask, and 2.1 g of phase transfer catalyst triethylphenylbenzyl ammonium chloride, 3.8 g of potassium borohydride, and 2.3 g of lithium carbonate were added. Reduction at 60°C for 6h. After the reaction was completed, it was cooled to 28°C, 10 g of KOH was direct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com