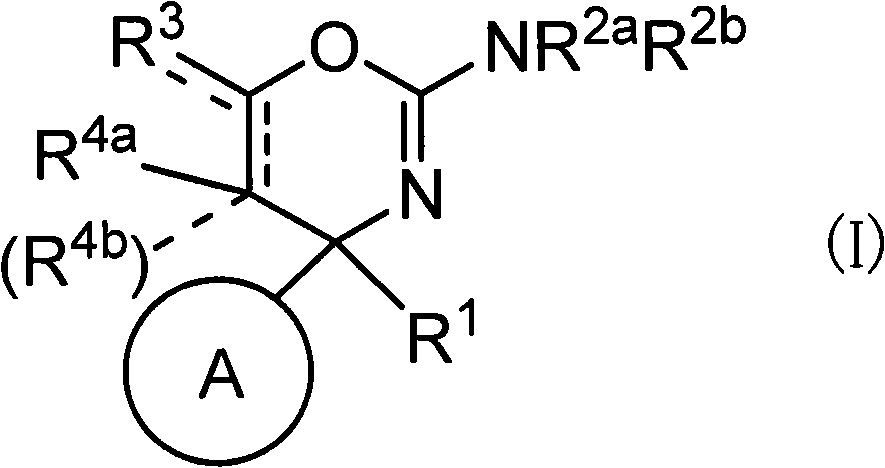
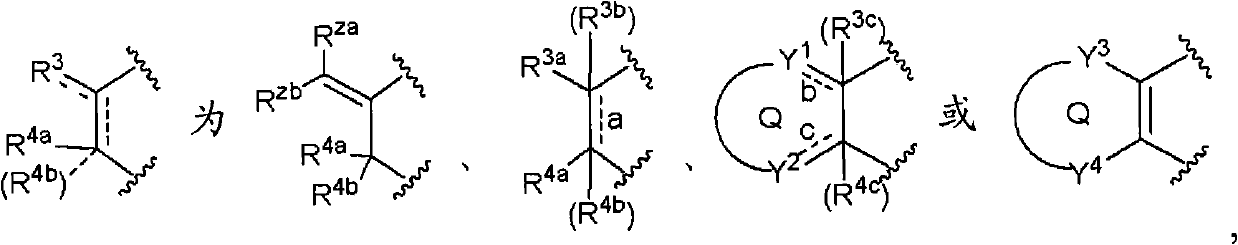
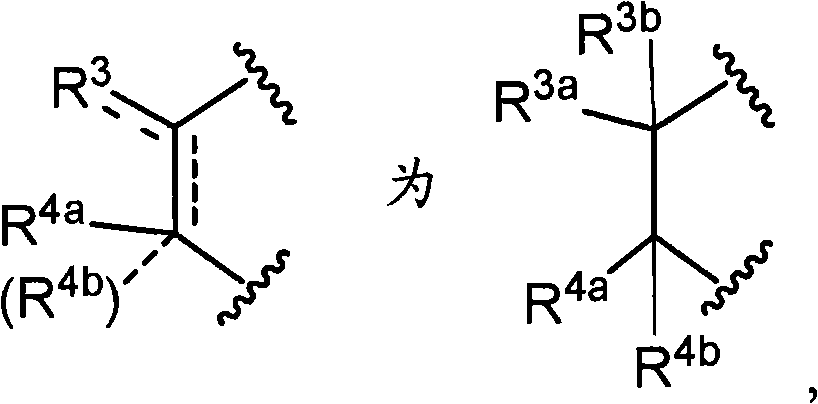
Oxazine derivative
A compound and solvate technology, applied in the field of oxazine derivatives, can solve the problem of no hint of drug activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0702] The synthesis of embodiment 1 compound (I-12) and (I-13)
[0703] [chemical formula 78]
[0704]
[0705] first step
[0706] To a solution of compound 1 (1.20 g) in acetone (70 ml)-water (40 ml) was added a solution of benzoyl isothiocyanate (0.82 g) in acetone (10 ml) at 0°C, and stirred at room temperature for 2 hours. After distilling off the solvent under reduced pressure, the residue was purified by column chromatography to obtain Compound 2 (1.35 g).
[0707] 1 H-NMR (CDCl 3 )δ: 1.69 (1H, t, J=4.7Hz), 2.14 (3H, s), 2.21-2.31 (1H, m), 2.73-2.83 (1H, m), 3.78-3.98 (2H, m), 7.15 (1H, dd, J=11.1, 9.1Hz), 7.48-7.55(2H, m), 7.60-7.67(1H, m), 7.85(2H, d, 7.2Hz), 8.14-8.20(1H, m), 8.30-8.34 (1H, m), 8.81 (1H, s), 11.56 (1H, s).
[0708] second step
[0709] Add methyl iodide (0.30ml) and diisopropylethylamine (0.84ml) to the acetonitrile (5ml) solution of compound 2 (1.26g) obtained in the first step, stir at room temperature for 32 hours, Stir for 2 hours. W...
Embodiment 2
[0717] Example 2 Synthesis of Compounds (I-14) and (I-15)
[0718]
[0719] first step
[0720] Benzoyl isocyanate (854 µl) was added to a tetrahydrofuran (10 ml) solution of compound (4) (1.1 g) obtained by the method described in Reference Example described later at 0°C, followed by stirring at room temperature for 30 minutes. Next, N-bromosuccinimide (675 mg) was added, followed by stirring at room temperature for 30 minutes. Ethyl acetate was added, washed with water and saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure to obtain compound (5) (2.4 g) as a crude product.
[0721] second step
[0722] Sodium tert-butoxide was added to a solution of compound (5) (2.4 g) obtained in the first step in tetrahydrofuran (12 ml) and dimethyl sulfide (12 ml), followed by stirring at room temperature for 2 hours. The reaction solution was poured into 1mol / L hydrochloric acid and extracted with ethyl acetate. The organic la...
Embodiment 3
[0740] The synthesis of embodiment 3 compound (I-54)
[0741]
[0742]
[0743] first step
[0744] Under a nitrogen atmosphere, compound (9) (3.00 g) prepared by the method described in WO2009 / 151098 was dissolved in tetrahydrofuran (30 ml), and cooled with a dry ice acetone bath. 2-Methallylmagnesium chloride (0.5 mol / L THF solution, 85.0 ml) was added dropwise at -78°C, followed by stirring at -78°C for 2 hours. Saturated ammonium chloride aqueous solution and water were added to the reaction liquid, extracted with ethyl acetate, washed with water and saturated brine in this order. After the organic layer was dried over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure, and the obtained residue was purified by column chromatography to obtain compound (11) (2.49 g).
[0745] 1 H-NMR (CDCl 3 )δ: 1.26(s, 9H), 1.38(s, 3H), 1.86(s, 3H), 2.84(ABq, J=13.4Hz, 2H), 4.21(s, 1H), 4.81(s, 1H), 4.92(d, J=1.5Hz, 1H), 7.05(dd, J=11.7, 8.7Hz, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com