Method for preparing ultrafine particles of water-insoluble or insoluble medicine
A technology of ultra-fine particles and drugs, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of precise control of drug particles, and achieve low cost, uniform and stable ultra-fine particles, Effect of improving solubility and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of ultrafine particles of itraconazole (ITA) by dispersing itraconazole (ITA) in hydroxypropylmethylcellulose (HPMC)
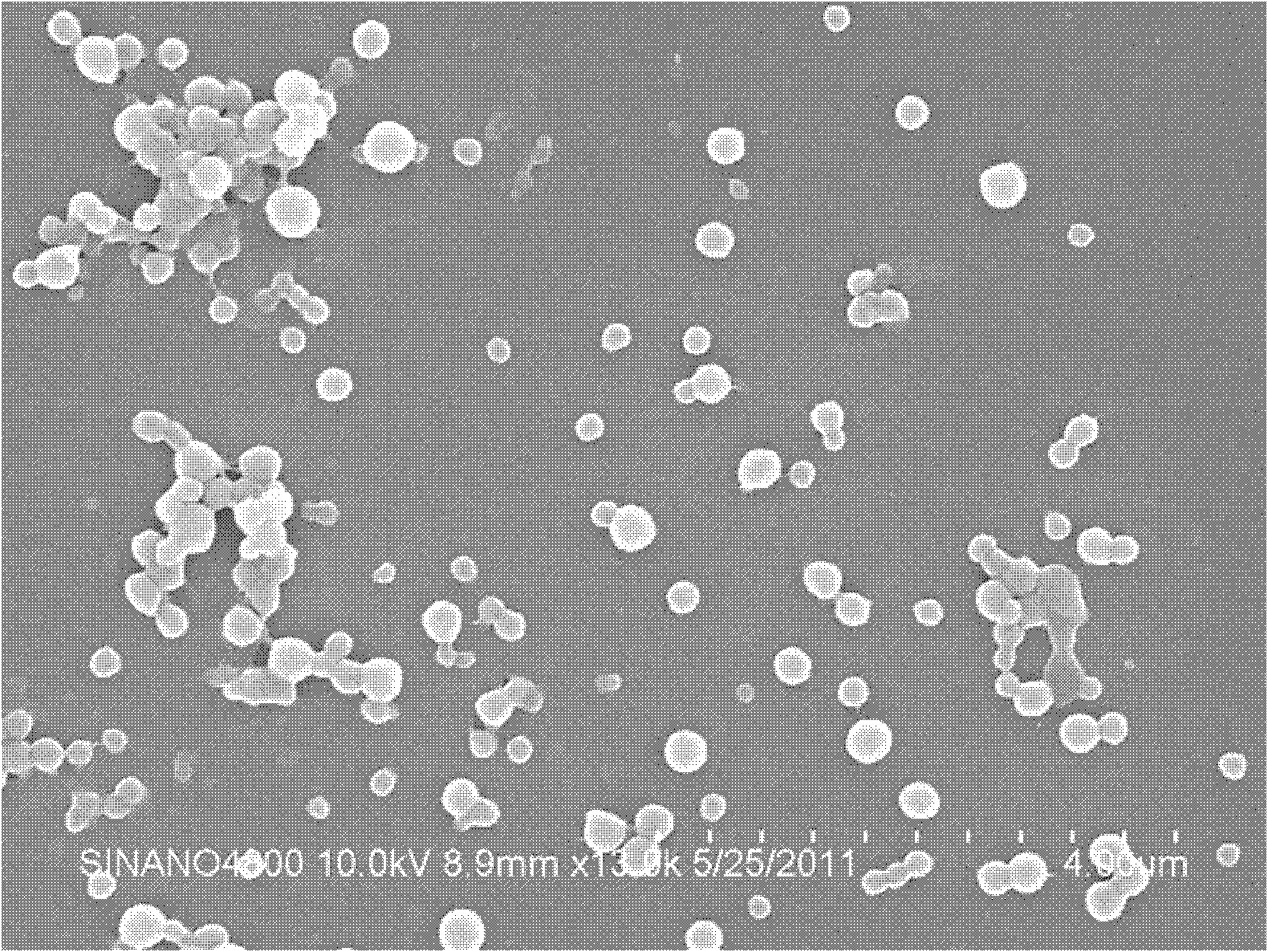
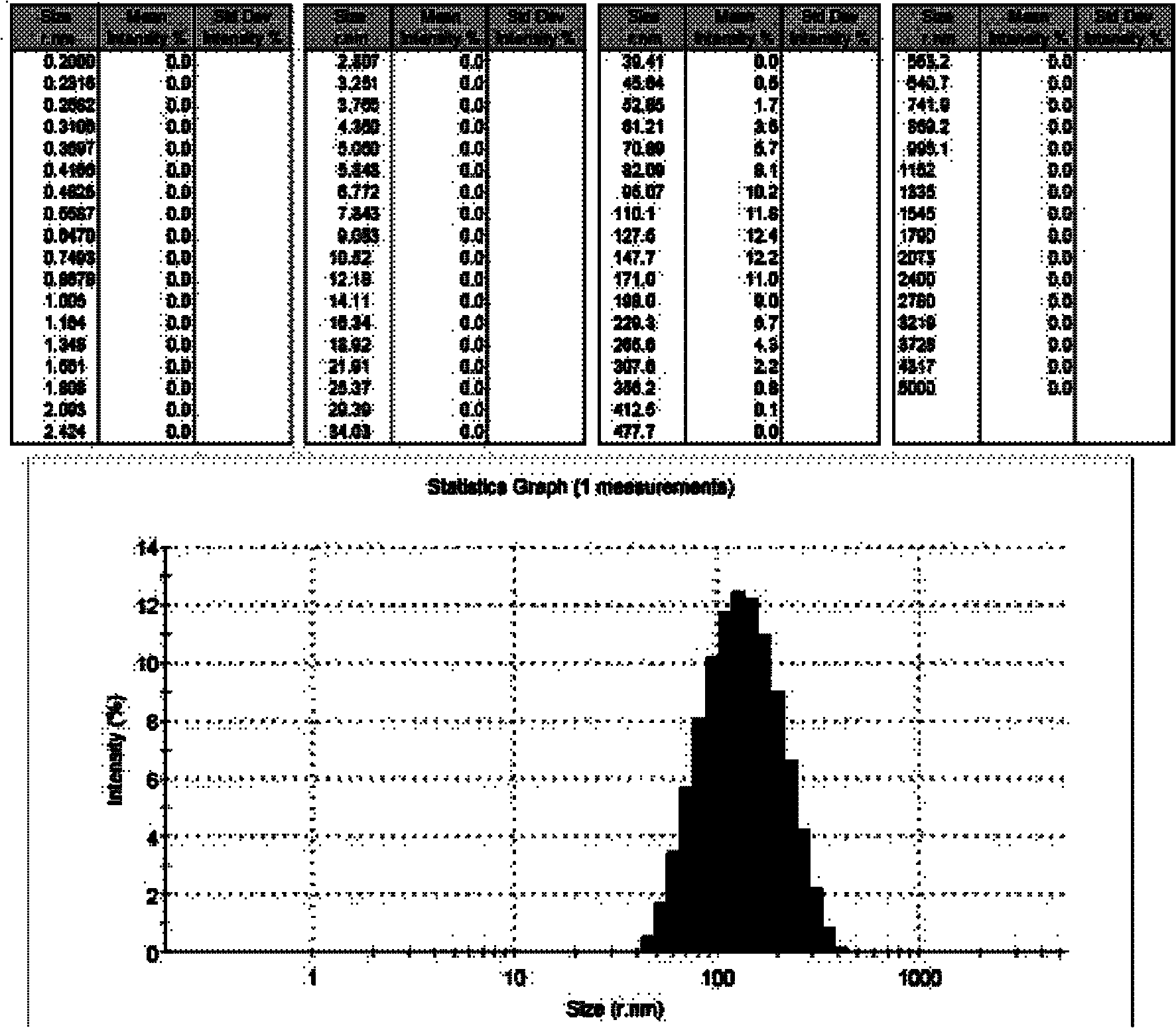
[0040] First, dissolve 0.5g of itraconazole (ITA) in 50ml of methanol / tetrahydrofuran (1:1) mixed solvent, and shake it for about 10 minutes to completely dissolve it to obtain solution A 1 ; Then prepare the 8.0mg / ml HPMC aqueous solution of 750ml, stir and make it mix homogeneously, get solution B 1 ; Then, at a stirring rate of 500rpm, the raw material solution A 1 Press into solution B through a single-channel PTFE membrane with an average pore size of 100 nm (POREFLON series from Sumitomo Electric, model: FP-010-60) 1 In the process, a white suspension of ultrafine particles is generated, and the stirring is continued for 2 minutes to make the reaction uniform; then the precipitate obtained is filtered with a 1.0 μm filter membrane to remove larger particles; finally, the slurry that produces ultrafine particles is freeze-d...
Embodiment 2
[0043] Example 2 Polyvinylpyrrolidone (PVP) disperses itraconazole (ITA) to prepare its ultrafine particles
[0044] First, dissolve 0.5g of itraconazole (ITA) in 50ml of methanol / tetrahydrofuran (1:1) mixed solvent, and shake it for about 10 minutes to completely dissolve it to obtain solution A 2 ; Then prepare the PVP aqueous solution of 8.0mg / ml of 750ml, stir and make it mix homogeneously, obtain solution B 2 ; Again, at a stirring rate of 500rpm, the raw material solution A 2 , pressed into solution B through a single-channel PTFE membrane with an average pore size of 100 nm (POREFLON series from Sumitomo Electric, model: FP-010-60) 2 In the process, a white ultrafine particle suspension is generated, and the stirring is continued for 2 minutes to make the reaction uniform; then the precipitate obtained is filtered with a 1.0 μm filter membrane to remove larger particles; finally, the slurry that produces particles is subjected to freeze-drying treatment or Spray dry...
Embodiment 3
[0047] Example 3 GC Headspace Sampling Method to Detect Residues of THF and Methanol in ITA Composite Nanoparticles
[0048] Use the GC headspace sampling method to detect the solvent residue in the ITA composite nanoparticles obtained in Implementation 1 and Example 2. The results show that the THF content is about 11ppm in ITA / HPMC, and the THF content in ITA / PVP is about 23.7ppm. Methanol was not detected.
[0049] Using GC headspace sampling method to detect the residues of tetrahydrofuran and methanol after ITA / HPMC were dried by different methods, the methanol peak represented 1000ppm, and the tetrahydrofuran peak represented 500ppm. The results showed that the residues of the two solvents were far lower than the pharmacopoeia upper limit concentration.
[0050] Through the comparison of the above experimental results, it can be seen that it is more appropriate to choose HPMC as the dispersant and freeze-drying as the condition, and the residual organic solvent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore diameter | aaaaa | aaaaa |
| Pore diameter | aaaaa | aaaaa |
| Pore diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com