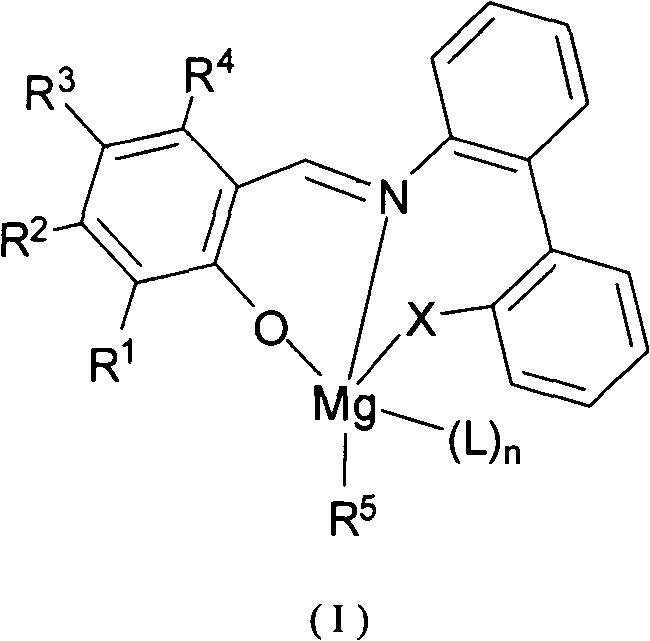
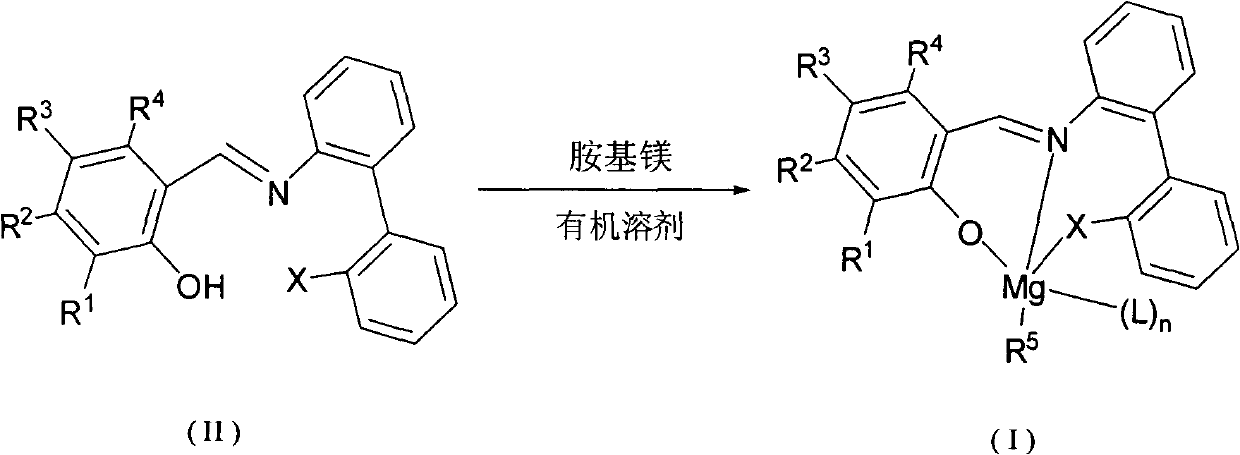
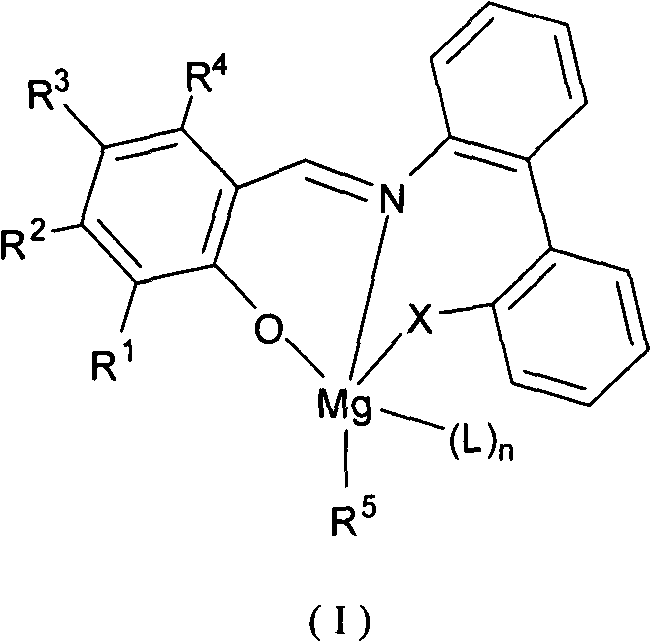
Imine phenol oxymagnesium compound, and preparation method and application thereof
A technology of iminophenoxymagnesium and compounds, which is applied in the field of iminophenoxymagnesium compounds, can solve the problem of rac-lactide inactivity, etc., and achieve the effects of convenient preparation, high catalytic activity and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of Ligand L1
[0037] Add 0.961g (5mmol) of 3-tert-butyl-5-methylsalicylaldehyde, 0.996g (5mmol) of 2-methoxy-2'-aminobiphenyl, 50mL of ethanol into a 100mL single-necked flask, at 80°C Heated to reflux for 3 h, concentrated to 20 mL, and recrystallized to obtain 1.494 g of orange-red crystals, with a yield of 80%.
[0038]
[0039] 1 H NMR (CDCl 3 , 400MHz): δ13.35(s, 1H, OH), 8.47(s, 1H, N-CH-Ar), 7.41(m, 2H, ArH), 7.34(t, 2H, 3 J=7.5Hz ArH), 7.22(m, 2H, ArH), 7.12(s, 1H, ArH), 7.00(t, 1H, 3 J=7.4Hz ArH), 6.96(m, 2H, ArH), 3.73(s, 3H, CH 3 O-Ar), 2.28(s, 3H, CH3-Ar), 1.37(s, 9H, C(CH 3 ) 3 ). 13 C NMR (CDCl 3 , 100MHz): δ162.77, 158.32, 156.46, 147.34, 137.19, 133.95, 131.29, 131.09, 130.98, 130.06, 128.97, 128.49, 128.41, 126.50, 126.42, 120.35, 118.7 (N-CH-Ar), 55.19 (CH 3 O-Ar), 34.70 (C (CH 3 ) 3 ), 29.23 (C (CH 3 ) 3 ), 20.62 (CH 3 -Ar).HRMS calcd.for C 25 h 27 NO 2 : 373.2042; found: 373.2041.
Embodiment 2
[0041] Synthesis of Ligand L2
[0042] Add 1.172g (5mmol) of 3,5-di-tert-butyl salicylaldehyde, 0.996g (5mmol) of 2-methoxy-2'-aminobiphenyl, and 50mL of ethanol into a 100mL single-necked flask, and heat to reflux at 80°C After 3 hours, it was concentrated to 20 mL and then recrystallized to obtain 1.704 g of orange-red crystals, with a yield of 82%.
[0043]
[0044] 1 H NMR (CDCl 3 , 400MHz): δ13.40(s, 1H, OH), 8.53(s, 1H, N-CH-Ar), 7.43-7.38(m, 3H, ArH), 7.34(m, 2H, ArH), 7.21( t, 2H, 3 J=6.7Hz ArH), 7.15(d, 1H, 4 J=2.3Hz ArH), 6.99(t, 1H, 3 J=7.4Hz ArH), 6.95(d, 1H, 3 J=8.3Hz ArH), 3.77(s, 3H, CH 3 O-Ar), 1.39(s, 9H, C(CH 3 ) 3 ), 1.31(s, 9H, C(CH 3 ) 3 ). 13 C NMR (CDCl 3 , 100MHz): δ163.06, 158.23, 156.47, 147.41, 140.00, 136.75, 134.05, 131.34, 130.96, 128.91, 128.50, 128.46, 127.57, 126.42, 126.37, 120.31, 118.3 (N-CH-Ar), 55.22 (CH 3 O-Ar), 35.01 (C (CH 3 ) 3 ), 34.10 (C (CH 3 ) 3 ), 31.45 (C (CH 3 ) 3 ), 29.27 (C (CH 3 ) 3 ).HRMS calcd.for ...
Embodiment 3
[0046] Synthesis of Ligand L3
[0047] Add 0.751g (5mmol) of 3,5-dimethyl salicylaldehyde, 0.996g (5mmol) of 2-methoxy-2'-aminobiphenyl, and 50mL of ethanol into a 100mL single-necked flask, and heat to reflux at 80°C for 3h , concentrated to 20 mL and then recrystallized to obtain 1.408 g of yellow crystals, with a yield of 85%.
[0048]
[0049] 1 H NMR (CDCl 3 , 400MHz): δ12.63(s, 1H, OH), 8.46(s, 1H, N-CH-Ar), 7.42-7.35(m, 4H, ArH), 7.24(dd, 1H, 3 J=7.4Hz, 4 J=1.7Hz ArH), 7.18(dd, 1H, 3 J=7.8Hz, 4 J=1Hz ArH), 7.01(m, 2H, ArH), 6.94(m, 2H, ArH), 3.71(s, 3H, CH 3 O-Ar), 2.26(s, 3H, CH 3 -Ar), 2.18(s, 3H, CH 3 -Ar). 13 C NMR (CDCl 3 , 100MHz): δ162.73, 157.14, 156.51, 147.78, 134.96, 133.74, 131.27, 131.15, 129.64, 129.09, 128.56, 128.49, 127.12, 126.39, 125.85, 120.50, 118.4 (N-CH-Ar), 55.35 (CH 3 O-Ar), 20.33 (CH 3 -Ar), 15.50 (CH 3 -Ar).HRMS calcd.for C 22 h 21 NO 2 : 331.1572; found: 331.1570.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conversion rate | aaaaa | aaaaa |
| Convert | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com