Solid preparation comprising pirfenidone as active component and application thereof
A technology of pirfenidone and solid preparations, which is applied in the field of medicine and can solve problems such as unclear drug target genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
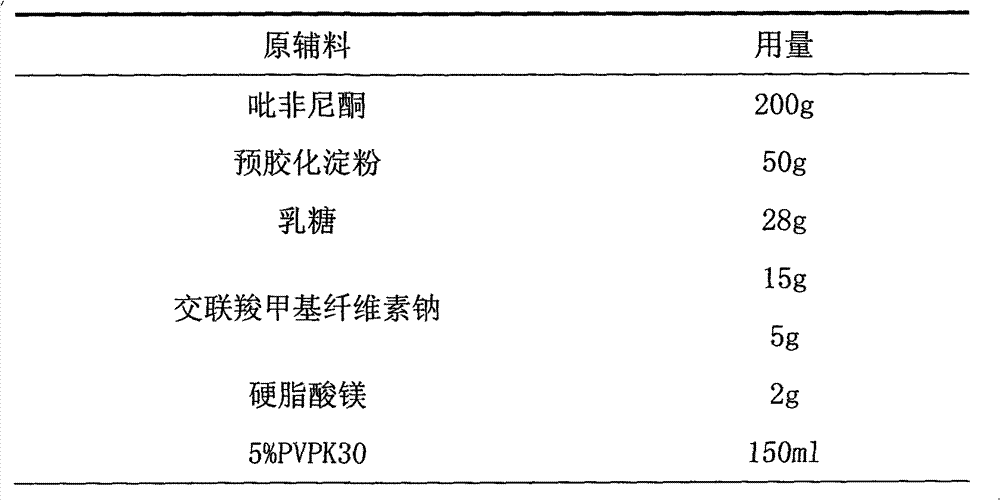
[0013] Make 1000 pirfenidone tablets with the raw materials of following weight ratio.
[0014]
[0015] Preparation Process:
[0016] 1. The pirfenidone raw material is crushed through an 80-mesh sieve, and set aside.
[0017] 2. Pass the lactose through a 60-mesh sieve and set aside.
[0018] 3. Take pirfenidone, pregelatinized starch, lactose, and internally added croscarmellose sodium and mix well.
[0019] 4. Make soft material with 5% PVPK30 solution, granulate with 20 mesh sieve, dry at 60°C, and granulate with 24 mesh sieve.
[0020] 5. Add external croscarmellose sodium and magnesium stearate, mix well, and press into tablets to get ready.
Embodiment 2
[0022] 1. Prepare 6% ethanol solution of Opadry enteric coating agent;
[0023] 2. Take the plain tablet obtained in Example 1, pour it into the coating pan, start the coating pan, and blow hot air, preheat the plain tablet at 30-40°C for 10 minutes, and blow off the drug powder adhering to the tablet core, Spray the coating solution evenly, so that the drug solution is evenly coated on the tablet core, and the pirfenidone enteric-coated tablet is obtained.
Embodiment 3
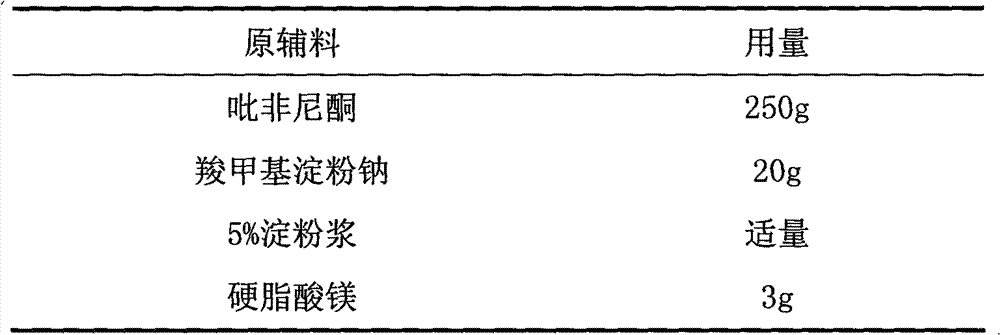
[0025] Make 1000 pirfenidone tablets with the raw materials of following weight ratio.
[0026]
[0027] Preparation:
[0028] 1. Grind Pirfenib and raw materials through an 80-mesh sieve, and set aside.
[0029] 2. Pass the lactose through a 60-mesh sieve and set aside.
[0030] 3. Take pirfenidone, pregelatinized starch, lactose and microcrystalline cellulose and mix well.
[0031] 4. Make soft material with water, granulate with 20 mesh, dry at 60°C, and granulate with 24 mesh.
[0032] 5. Add croscarmellose sodium and magnesium stearate, mix well, and press into tablets to get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com