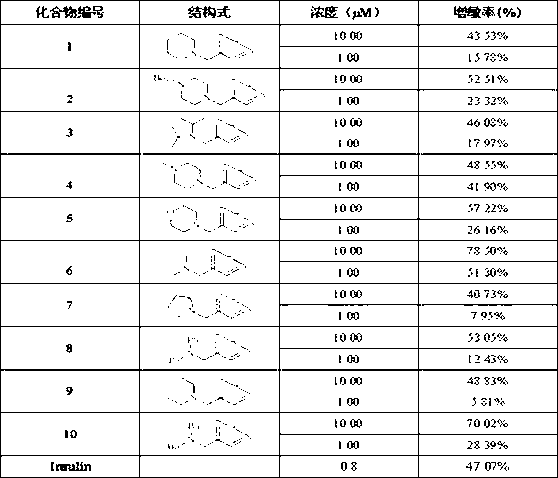
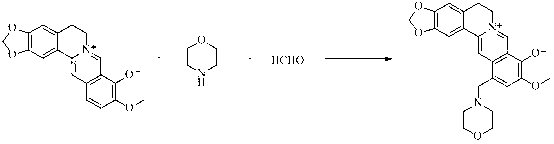
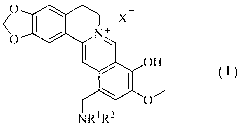
Berberrubine 12-site derivatives as well as preparation method and applications thereof
A technology of berberine and aminomethylberberine, which is applied in the field of medicinal chemistry and drug therapy, and achieves the effect of simple synthesis and abundant synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of Berberine
[0026]
[0027] Put 20g of dry berberine hydrochloride in a round bottom flask, vacuumize to a pressure of 10mmHg, and react at 180°C for 20min until the raw material completely changes from yellow to dark red. The obtained crude product was eluted with dichloromethane / methanol (V / V=10:1) column chromatography to obtain 22 g of red solid powder.
[0028] 1 H NMR (400 MHz, DMSO-d 6 ,): δ 9.22 (s, 1H), 8.06 (s, 1H), 7.56 (d, 1H, J = 8 Hz), 7.43 (s, 1H), 6.96 (s, 1H), 6.85 (d, 1H, J = 8 Hz), 6.10 (s, 2H), 4.70 (t, J = 6 Hz, 2H), 3.78 (s, 3H), 3.23 (t, J = 6 Hz, 2H). MS (ESI) m / z: 322 [M+H] + .
Embodiment 2
[0030] Preparation of 12-(N,N-dimethylamino-methyl)-berberine
[0031]
[0032]Add 322mg (1mmol) of berberine hydrochloride and 5ml of absolute ethanol to the reaction bottle, add 0.66ml of aqueous dimethylamine (33%) (5mmol) and 0.4ml of aqueous formaldehyde (37%) (5mmol) under stirring at room temperature, React at 80°C for 24 hours. At the end of the reaction, the solvent was concentrated under reduced pressure and subjected to flash column chromatography, eluting with dichloromethane: methanol (V / V = 20: 1), to obtain 286 mg of a dark red solid.
[0033] 1 H NMR (400 MHz, DMSO-d 6 ,): δ 9.07 (s, 1H), 7.98 (s, 1H), 7.64 (s, 1H), 7.13 (s, 1H), 6.96 (s, 1H), 6.10 (s, 2H), 4.47 (t, J = 6 Hz, 2H), 3.72 (s, 3H), 3.56 (s, 2H), 3.03 (t, J = 6 Hz, 2H), 2.16 (s, 6H). MS (ESI) m / z: 379 [M+H] + .
Embodiment 3
[0035] Preparation of 12-(pyrroline-1-methyl)-berberubine
[0036]
[0037] Add 322mg (1mmol) of berberine hydrochloride and 5ml of absolute ethanol to the reaction bottle, add 0.42ml of tetrahydropyrrole (5mmol) and 0.4ml of formaldehyde aqueous solution 0.4 (37%) (5mmol) under stirring at room temperature, and react at room temperature for 24 Hour. After the reaction, the solvent was concentrated and dried under reduced pressure, followed by flash column chromatography, eluting with dichloromethane: methanol (V / V = 20: 1), to obtain 312 mg of a dark red solid.
[0038] 1 H NMR (400 MHz, CDCl 3 ): δ 9.17 (s, 1H), 8.01 (s, 2H), 7.26 (s, 2H), 6.74 (s, 1H), 6.05 (s, 2H), 4.36 (t, J = 6 Hz, 2H), 3.93 (s, 3H), 3.78 (s, 2H), 3.05 (t, J = 6 Hz, 2H), 2.55 (s, 4H), 1.68 (s, 4H). MS (ESI) m / z: 406 [M+H].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com