Derivative of Dysosma versipellis, and composition, preparation method and use thereof
A technology of derivatives and compounds, applied in the field of podophyllotoxin derivatives and compositions thereof, can solve the problems of interfering with DNA replication and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
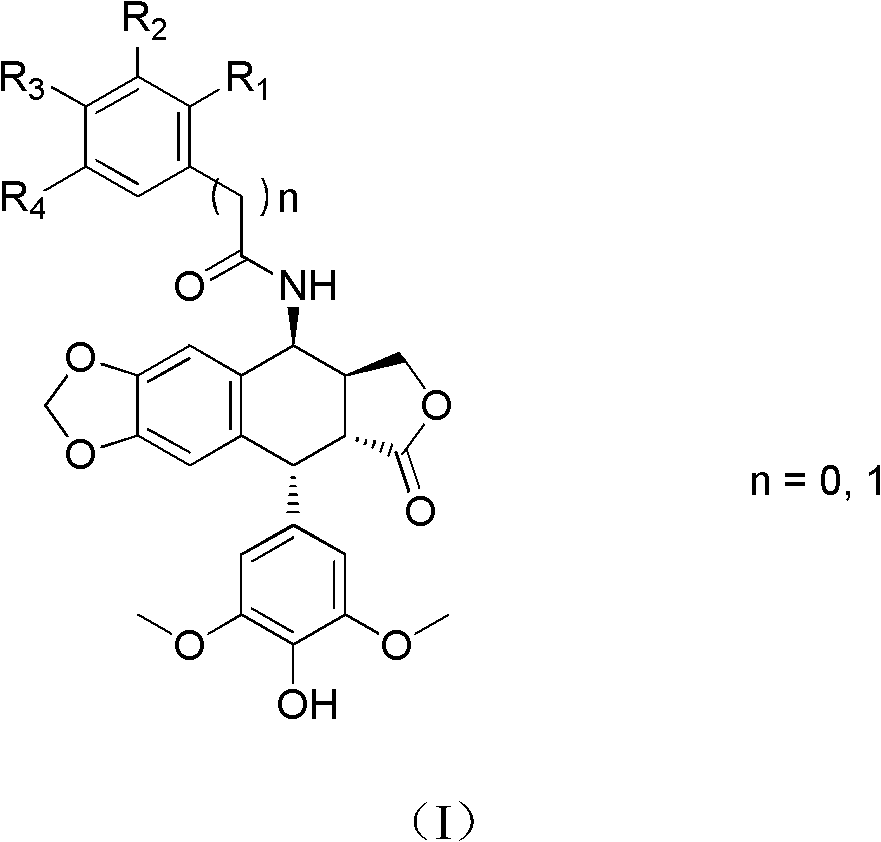
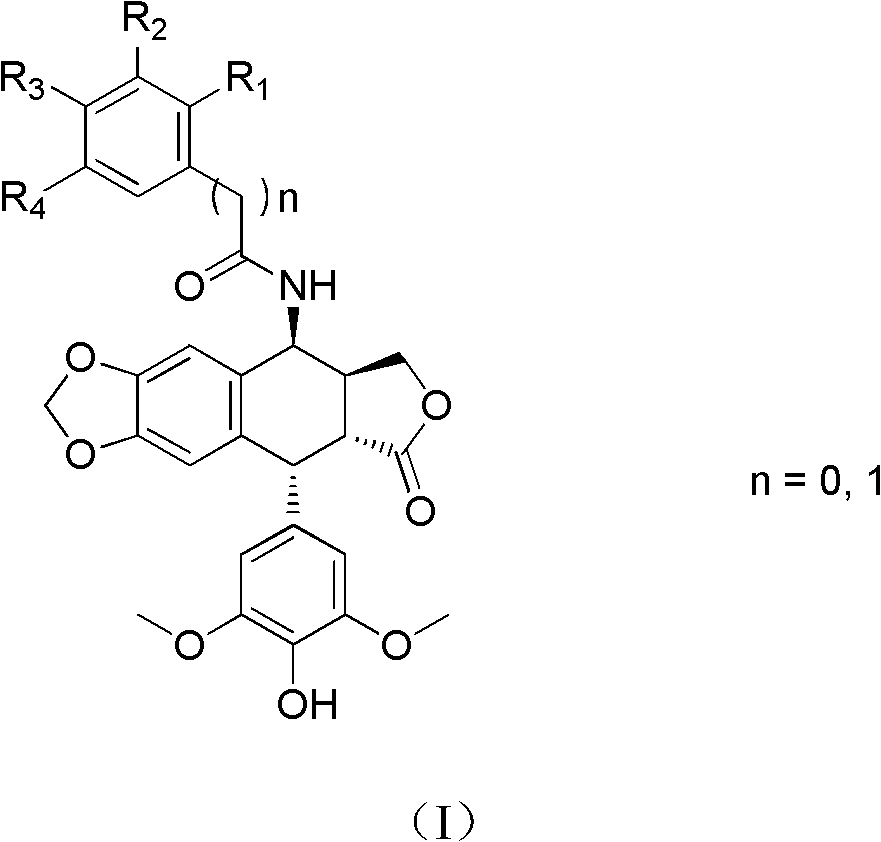
[0062] N-((5S, 5aS, 8aR, 9R)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexa Preparation of hydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxo-5-cycl-5-yl)-3-trifluoromethylbenzamide ( Compound 1)
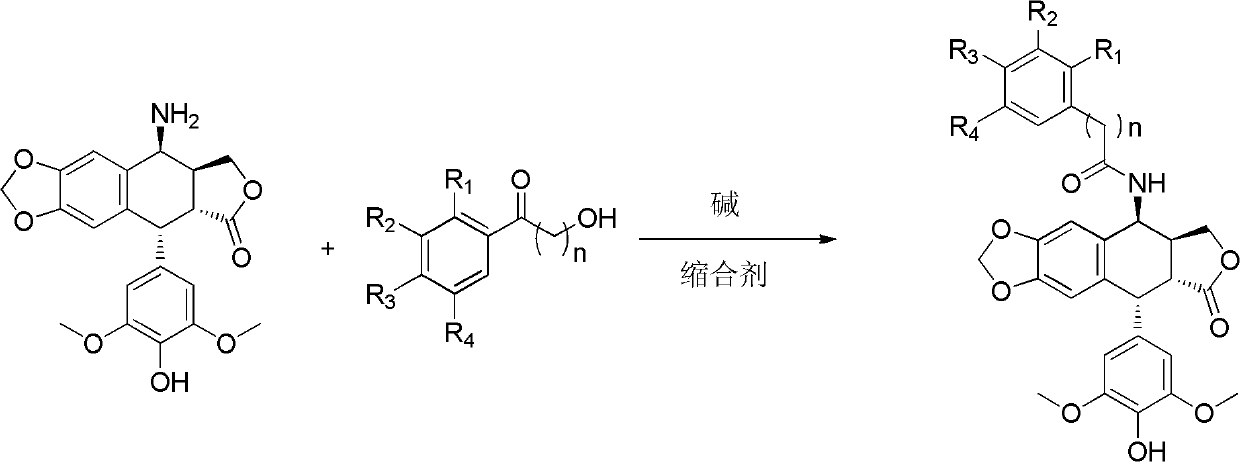
[0063] 3-Trifluoromethylbenzoic acid (475mg, 2.5mmol), 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDCI) (525mg, 2.76mmol ), 1-hydroxybenzotriazole (350mg, 2.6mmol), were added into dichloromethane (10ml), stirred for 30min, then compound II (1.0g, 2.5mmol) was added, and reacted at 25-30°C for 4h. The reaction solution was washed with water and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and the obtained crude product was purified by column chromatography (eluent: dichloromethane / acetone gradient elution) to obtain compound 1 (728 mg) as a white solid. Melting point: 168.9-173.3°C. ESI-MS: 572 (M + +H), 594(M + +Na), 610(M + +K). 1 HNMR / (CDCl 3 ) δppm: 8.051 (1H, s, Ar-H), 7.979 (1H, d, ...
Embodiment 2
[0065] 2-Chloro-N-((5S, 5aS, 8aR, 9R)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a , 9-Hexahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxacyclo-5-yl)-3,4-dimethoxy Preparation of benzamide (compound 2)
[0066] 2-Chloro-3,4-dimethoxybenzoic acid (216 mg, 1 mmol), 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDCI) (191 mg, 1 mmol), 1-hydroxybenzotriazole (135 mg, 1 mmol), were added to dichloromethane (5 ml). After stirring for 2h, compound II (399mg, 1mmol) was added and reacted at 25-30°C for 4h. The reaction solution was washed with water and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and the obtained crude product was purified by column chromatography (eluent: dichloromethane / acetone gradient elution) to obtain compound 2 (308 mg) as a white solid. Melting point: 132.1~136.2℃. ESI-MS: 598 (M + +H), 620(M + +Na), 1217 (2M + +Na). 1 HNMR / (CDCl 3 )δppm: 7.490 (1H, d, J = 8.4Hz, Ar-H)...
Embodiment 3
[0068] N-((5S, 5aS, 8aR, 9R)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexa Hydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxacyclo-5-yl)-2,3,4-trimethoxybenzamide (Compound 3) Preparation
[0069] 2,3,4-trimethoxybenzoic acid (212mg, 1mmol), 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDCI) (191mg, 1 mmol), 1-hydroxybenzotriazole (135 mg, 1 mmol), was added to dichloromethane (5 ml). After stirring for 2h, compound II (399mg, 1mmol) was added and reacted at 25-30°C for 4h. The reaction solution was washed with water and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and the resulting crude product was purified by column chromatography (eluent: dichloromethane / acetone gradient elution) to obtain compound 3 (298 mg) as a white solid. Melting point: 137.4-141.9°C. ESI-MS: 594 (M + +H), 616(M + +Na), 632 (M + +K). 1 HNMR / (CDCl 3 )δppm: 8.078 (1H, d, J = 6.8Hz, NH), 7.868 (1H, d, J = 9.2Hz,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com