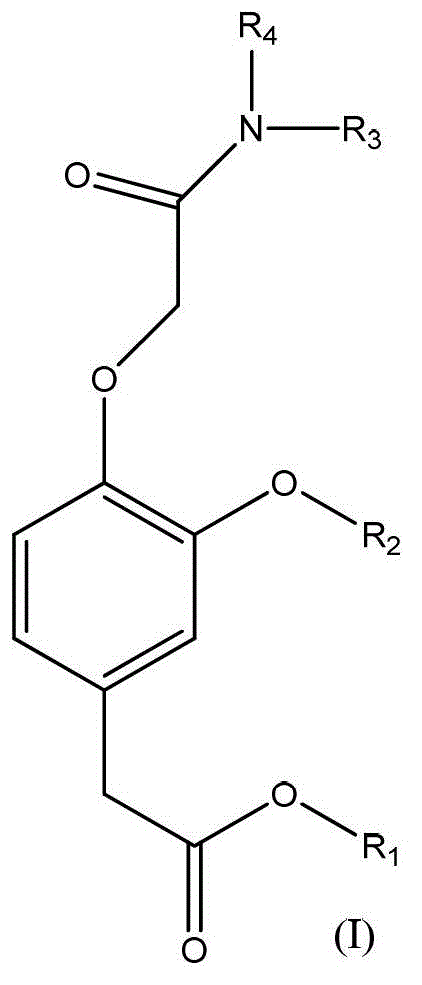
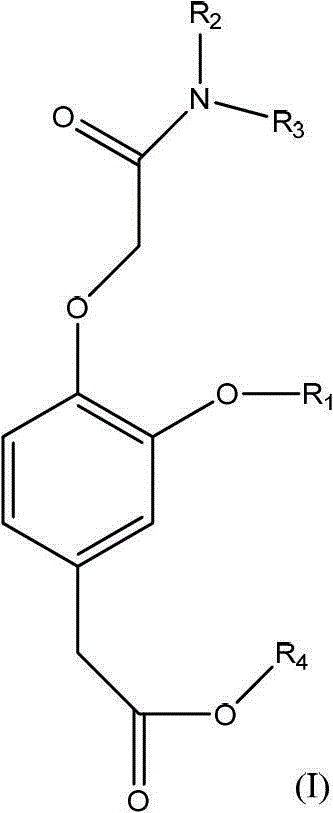
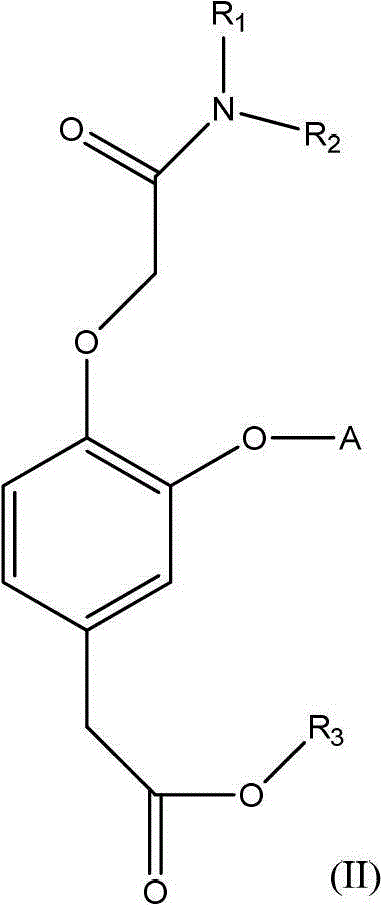
Substituted phenyl acetate compound and application thereof
A technology of compound and alkyl, which is applied in the field of medicine and chemistry to achieve the effect of strong drug effect, less toxic and side effects, and good anesthesia effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1.1, the synthesis process of (3-trifluoromethoxy-4-hydroxyl) trifluoroethyl phenylacetate (intermediate compound a) The intermediate compound a can be prepared by the following reaction process:
[0049]
[0050] 1.1.1 Intermediate compound a 2 synthesis process
[0051] 2-(trifluoromethoxy)phenol (intermediate compound a 1 ) (35.625g, 0.2mol, 1eq) and 50% glyoxylic acid (29.5g, 0.198mol, 1eq) and 100ml of distilled water were added to a 500ml three-necked flask, and 10% NaOH solution was added dropwise to the there-necked flask under an ice bath (prepared by 16.1g sodium hydroxide and 150ml distilled water), react at room temperature overnight. Stop the reaction, extract 3 times with ethyl acetate, adjust the pH of the aqueous phase to 1 with concentrated hydrochloric acid, extract 3 times with ethyl acetate, dry the organic phase with anhydrous magnesium sulfate, spin dry the solvent with a rotary evaporator, and obtain 53.6 g of a white solid , yield 53%, mp 1...
Embodiment 2
[0061]2.1 Synthetic process of 3-trifluoroethoxy-4-phenylacetic acid trifluoroethyl ester (intermediate compound b)
[0062] Using 2-(2,2,2-trifluoroethoxy)phenol instead of 2-(trifluoromethoxy)phenol, it was prepared using a similar process to Example 1.1. The intermediate compound b4.7g was obtained. 1 H-NMR (CDCl 3 )δ3.67 (s 2H), 4.42-4.52 (m, 4H), 5.35 (s, 1H), 6.95 (s, 3H). MS (ESI) m / z 333 ([M+H] + ).
[0063]
[0064] 2.2 Synthetic process of [4-[(N,N-ethylcarbamoyl)methoxy]-3-trifluoroethoxyphenyl]trifluoroethyl acetate (compound 2)
[0065] In a 100 ml round bottom flask equipped with a magnetic stirring bar, intermediate compound b (4.7 g, 14 mmol, 1.0 eq) was dissolved in anhydrous acetone (50 ml) to form a solution. To this solution was added potassium carbonate (3 g, 21 mmol, 1.5 eq) followed by 2-chloro-N,N-diethylacetamide (2.53 g, 16.8 mmol, 1.2 eq). Under magnetic stirring, the suspension was heated to reflux and kept under reflux for 48h. After cooli...
Embodiment 3
[0068] 3.1 Synthetic process of 3-methyl-4-phenylacetic acid trifluoroethyl ester (intermediate compound c)
[0069] Using guaiacol instead of 2-(trifluoromethoxy)phenol, it was prepared using a similar process to Example 1.1. The intermediate compound c3.6g was obtained. 1 H-NMR (CDCl 3 )δ3.67(s 2H), 3.88(s, 3H), 4.46-4.53(m, 2H), 5.35(s, 1H), 6.78-6.86(m, 2H), 6.92(d, 1H, J=4Hz ).MS (ESI) m / z 265 ([M+H] + ).
[0070]
[0071] 3.2, [4-[(N, N-ethylcarbamoyl) methoxyl]-3-methoxyphenyl] trifluoroethyl acetate (compound 3) synthesis process in the 100ml equipped with a magnetic stirring bar Intermediate compound c (3.6g, 13.6mmol, 1.0eq) was dissolved in anhydrous acetone (50ml) to form a solution in a round bottom flask. To this solution was added potassium carbonate (3g, 21.7mmol, 1.5eq) followed by 2-chloro-N,N-diethylacetamide (2.4g, 16mmol, 1.2eq). Under magnetic stirring, the reaction solution was heated to reflux and kept under reflux for 48h. After cooling to ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com