CD20-resistant monoclonal antibody as well as preparation method and application thereof
A monoclonal antibody and nucleic acid molecule technology, applied in the field of biomedicine, can solve the problems of high cost of drug treatment and difficult for patients to receive treatment, and achieve the effect of strong pharmacological effect and safe use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
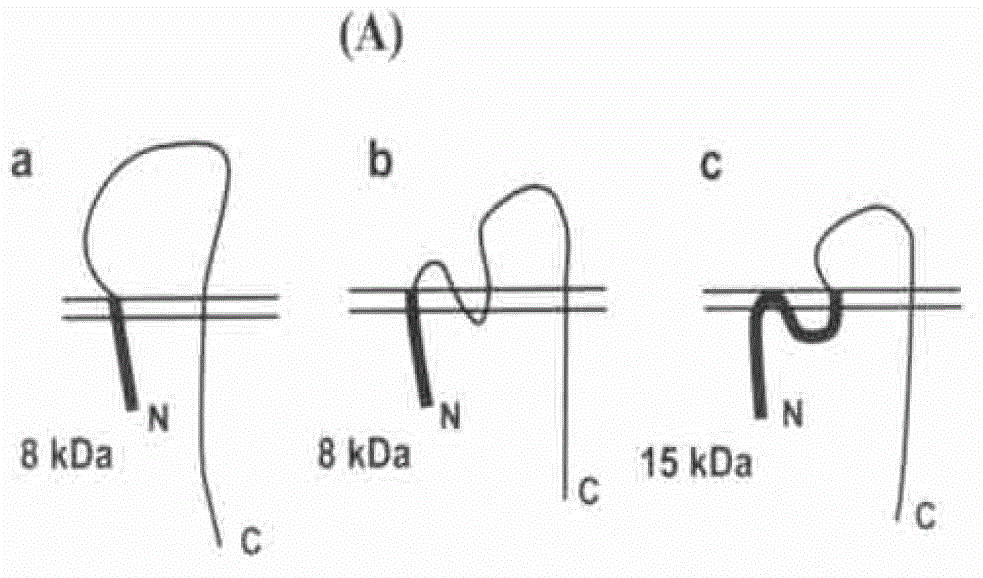
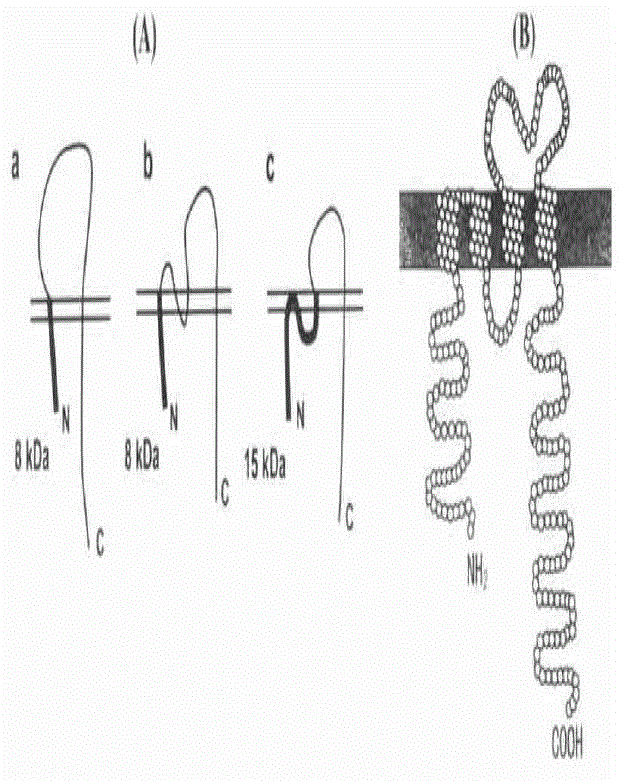
Image
Examples
Embodiment 1
[0062] Example 1 Preparation and Screening of Mouse Monoclonal Antibody
[0063] (1) Preparation of antigen The human B lymphocyte-specific membrane antigen CD20 gene was amplified from the human gene transcriptome by PCR. The primers were synthesized by Shanghai Sangon Bioengineering Co., Ltd. The sequence is as follows:
[0064] GSP1-H, 5'-ACC TGG GTAGGT GCG GAC ACC ACT-3';
[0065] GSP2-H, 5'-CAC ACT TTC ACG TCAAGG ACA-3';
[0066] GSP3-H,5'-CTT GTC CAG GGA TCC TAG AGT-3';
[0067] GSP1-L,5'-TTG CAG TCC TGA TCA GGC CAA CT-3';
[0068] GSP2-L,5'-TGT CGT GCA CTG GCAACA ATC TT-3':
[0069] GSP3-L,5'-TTG TTC ATG AAG CTC AAG GCT GA-3'.
[0070] Using BamHI and Xho I double enzyme digestion, the gene fragment was connected into the prokaryotic expression vector pGEM-T (promega company product of the United States), PCR and double enzyme digestion identified positive clones and then sequenced. After the sequence was determined correctly, the plasmid was extracted. Prokaryotic...
Embodiment 2
[0088] Example 2 Humanization and Expression Purification of Mouse Monoclonal Antibody
[0089] (1) Humanization of mouse monoclonal antibody The amino acid sequence of the mouse antibody was compared with the amino acid sequence of the human antibody, and the structure of the consensus sequence of the human subclass with the highest homologous sequence was obtained, that is, the human light chain VLκ subgroup II ( VLκII) and heavy chain VH subgroup I (VHI), as humanized frameworks. According to the definition of kabat et al., CDR1:24-34, CDR2:50-56, CDR3:89-97 of the L chain; CDR1:26-35, CDR2:50-65, CDR3:95-102 of the H chain (sequences of proteins of immunological Interest, (5th), Public Health Service, National Institutes of Health, Bethesda, MD (1991). Utilize computer to construct the three-dimensional model of mouse monoclonal antibody VL-VH region (Carter etc., Proc.Natl.Acad. Sci. USA 89: 4285-4289 (1992) Eigenbrot. J. Mol. Biol.) 229: 969-995 (1993)), determining whi...
Embodiment 3
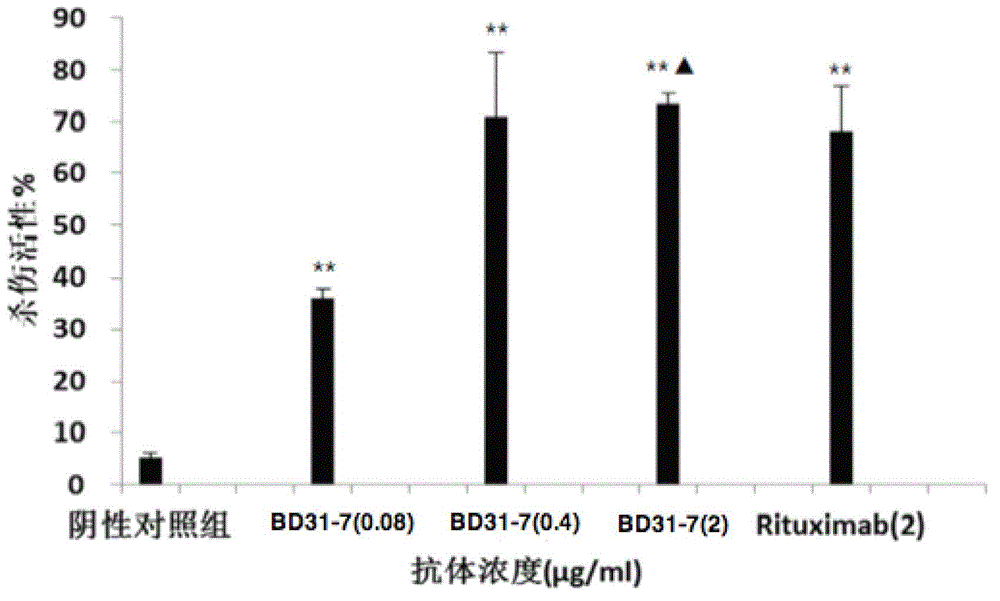
[0091] Example 3 Affinity Analysis of Humanized Monoclonal Antibody CD20
[0092] The affinity of monoclonal antibody 31-7, BD31-7 and Rituximab was determined by Scatchard method. Table 1 shows the results of the assay, and the experimental results show that the two monoclonal antibodies have strong binding affinity to VEGF.
[0093] Table 1 Monoclonal antibody affinity results
[0094]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com