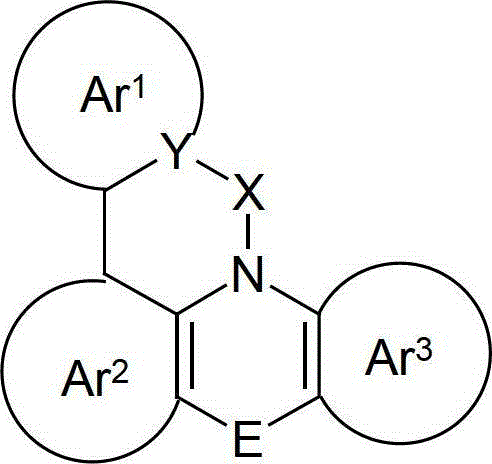
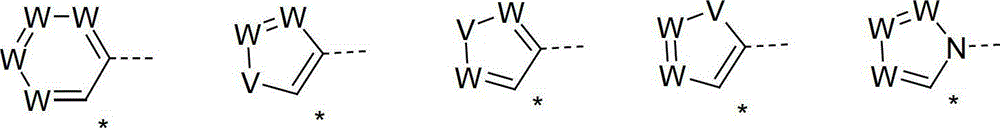
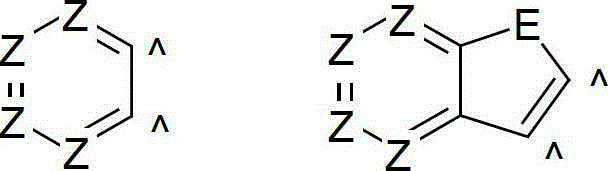Organic electroluminescence devices
A technology of electronic devices and general formulas, applied in the field of materials for organic electroluminescent devices, can solve problems affecting material preparation, unfavorable, strong oxidation sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0200] Example 1a: 3-Phenyl-4H-furo[3,4-b]indole
[0201]
[0202] 45 g (123 mmol) 3-phenyl-4-(benzenesulfonyl)-4H-furo[3,4-b]indole and 48 g (856 mmol) potassium hydroxide in 65 ml DMSO and 21 ml water were heated under reflux 1 hour. The mixture was then cooled to room temperature, neutralized with 1M HCl solution and extracted with dichloromethane. The solvent was evaporated under vacuum and the residue was purified by chromatography (heptane / ethyl acetate 10:1). Yield: 22.4 g (96 mmol), 80%.
[0203] The following compounds are similarly obtained:
[0204]
Embodiment 2a
[0205] Example 2a: 2-Bromophenyl-(3-phenylfuro[3,4-b]indol-4-yl)methanone
[0206]
[0207] 2.1 g (52.5 mmol) NaH (60% in mineral oil) were dissolved in 500 ml THF under a protective atmosphere. 11.5 g (50 mmol) of 3-phenyl-4H-furo[3,4-b]indole and 11.5 g (52.5 mmol) of 15-crown-5 dissolved in 200 ml of THF were added. After 1 hour at room temperature, a solution of 12 g (55 mmol) of 2-bromobenzoyl chloride in 250 ml of tetrahydrofuran was added dropwise. The reaction mixture was stirred at room temperature for 18 hours. After this time, the reaction mixture was poured onto ice and extracted three times with dichloromethane. Use Na 2 SO 4 The combined organic phases were dried and evaporated. The residue was extracted with hot toluene and recrystallized from toluene / n-heptane. Yield was 12 g (60%).
[0208] The following compounds are similarly obtained:
[0209]
[0210]
Embodiment 2i
[0211] Example 2i: (1-Chlorobenzo[4,5]furo[3,2-b]indol-10-yl)phenyl ketone
[0212]
[0213] 2.1 g (52.5 mmol) NaH (60% in mineral oil) were dissolved in 500 ml THF under a protective atmosphere. 12.6 g (50 mmol) of 3-phenyl-4H-furo[3,4-b]indole and 11.5 g (52.5 mmol) of 15-crown-5 dissolved in 200 ml of THF were added. After 1 hour at room temperature, a solution of 7.7 g (55 mmol) of benzoyl chloride in 250 ml of THF was added dropwise. The reaction mixture was then stirred at room temperature for 18 hours. After this time, the reaction mixture was poured onto ice and extracted three times with dichloromethane. Use Na 2 SO 4 The combined organic phases were dried and evaporated. The residue was extracted with hot toluene and recrystallized from toluene / n-heptane. Yield was 12.5 g (69%).
[0214] The following compounds are similarly obtained:
[0215]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com