Latamoxef Sodium midbody synthetic method
A technology of latamoxef sodium and a synthetic method, which is applied in the field of preparation of latamoxef sodium, can solve the problems of being unsuitable for industrial production, increasing uncontrollable reaction, and low yield, so as to improve equipment utilization and reduce economic burden , the effect of improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
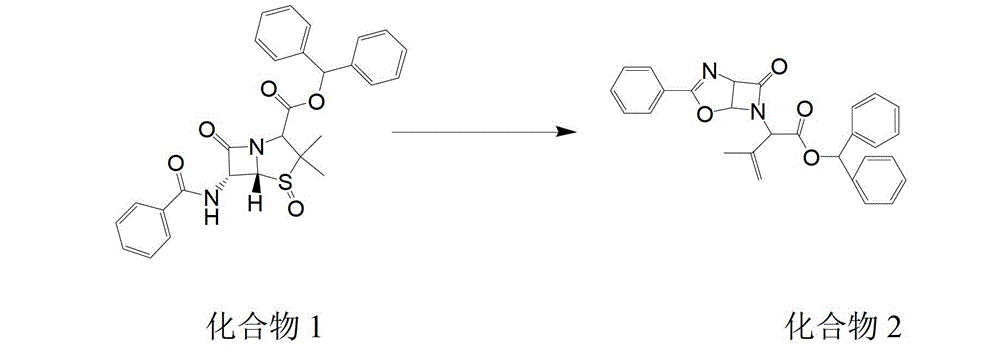
[0026] see figure 1 , a kind of synthetic method of laoxef sodium intermediate, comprises the following steps:
[0027] 1) Add 150g of toluene, 30g of compound 1 and 12g of triethyl phosphite in sequence to a dry and clean 1L four-necked bottle. After setting up the reflux device, turn on the cooling water and control the internal temperature at 110-120℃, so that the material is refluxed. The reaction is carried out under the state for 4-5h;
[0028] 2) In the above reaction, when the residue of compound 1 is less than or equal to 0.5%, the reaction is completed, then at 50 ~ 55 ℃, reduce the pressure to -0.08MPa and concentrate to evaporate to dryness, then add 300g methanol to the evaporated concentrate, and cool down. To 0 ~ 10 ℃, grow crystals for 1 ~ 2h.
[0029] 3) After the crystal growth is completed, filter, rinse with methanol at 0-5°C, and dry at 50-60°C under negative pressure -0.08MPa for 8-12h to obtain compound 2.
[0030] Using triethyl phosphite as the cata...
Embodiment 2
[0032] A method for synthesizing a laoxef sodium intermediate, comprising the following steps:
[0033] 1) Add 120g of toluene, 30g of compound 1 and 6g of triethyl phosphite in sequence to a dry and clean 1L four-necked bottle. After setting up the reflux device, turn on the cooling water and control the internal temperature at 80-100℃, so that the material is refluxed. The reaction is carried out under the state for 4 to 6 hours;
[0034] 2) When the residue of the above reaction (compound 1) is less than or equal to 0.5%, the reaction is completed, then at 45 ~ 55 ℃, reduce the pressure to -0.08MPa and concentrate to evaporate to dryness, and then add 200g isopropanol to the evaporated concentrate , cooled to 0 ~ 5 ℃, crystal growth for 1.5h.
[0035] 3) After the crystal growth is completed, filter, rinse with isopropanol at 0-5°C, and dry at 50-60°C under negative pressure -0.08MPa for 8-10h to obtain compound 2.
Embodiment 3
[0037] A method for synthesizing a laoxef sodium intermediate, comprising the following steps:
[0038] 1) Add 120g of benzene, 30g of compound 1 and 5g of triethyl phosphite in sequence to a dry and clean 1L four-necked bottle. After setting up the reflux device, turn on the cooling water and control the internal temperature at 80-100℃, so that the material is refluxed. The reaction is carried out under the state for 4 to 6 hours;
[0039] 2) When the residue of compound 1 in the above reaction is less than or equal to 0.5%, the reaction is completed, then at 45 ~ 55 ℃ under reduced pressure -0.08MPa concentrated to evaporate to dryness, then add 200g ethanol to the evaporated concentrate, cool down to 5 ~10℃, grow crystals for 2h.
[0040] 3) After the crystal growth is completed, filter, rinse with ethanol at 0-5°C in a freezer, and dry at 50-60°C under negative pressure -0.08MPa for 8-12 hours to obtain compound 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com