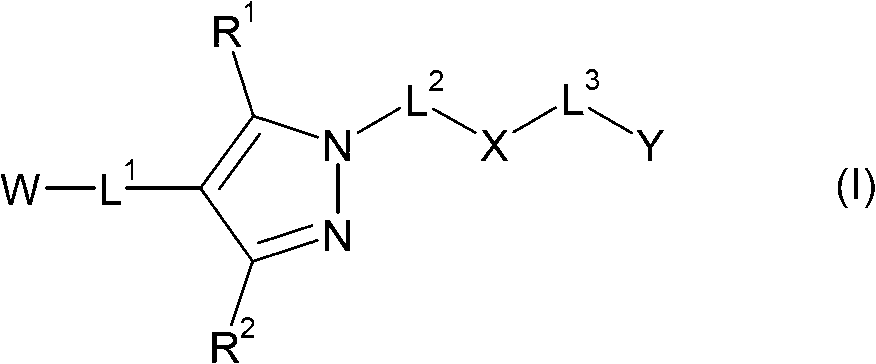
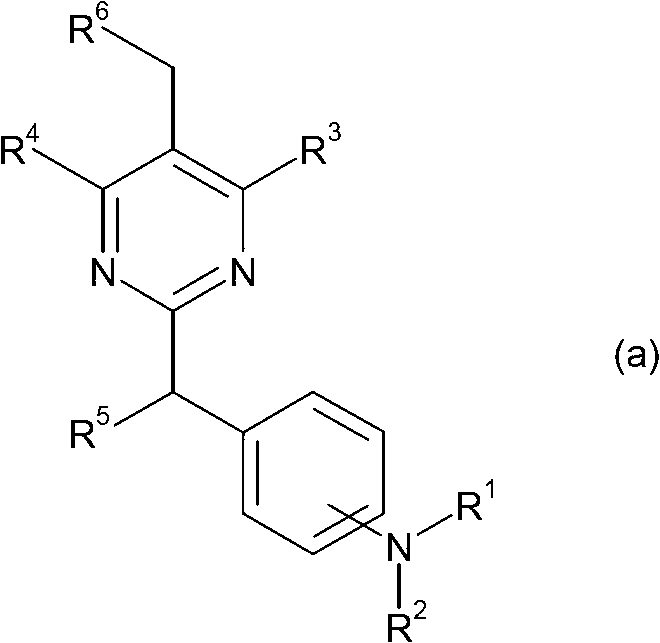
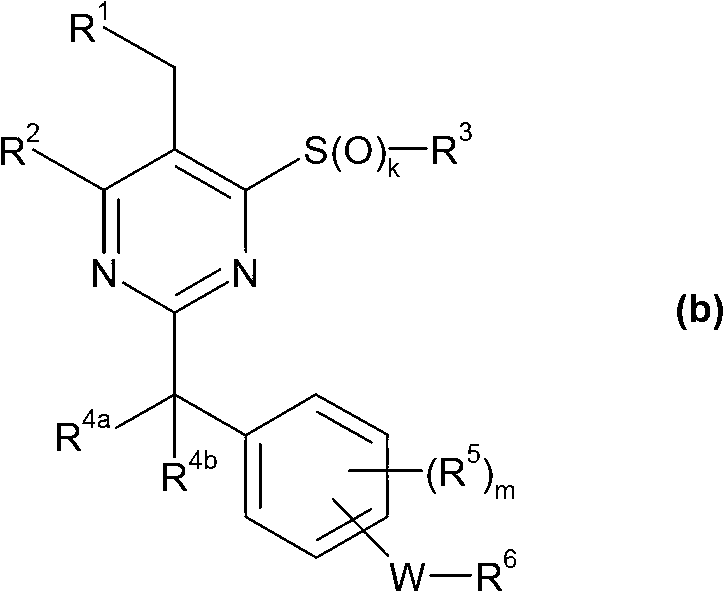
Pyrazole compounds as CRTH2 antagonists
A compound, pyrazole technology, applied in the direction of anti-inflammatory agent, drug combination, organic chemistry, etc., can solve the problem of not promoting inflammatory response and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0239] {3,5-Dimethyl-1-[4-(4-trifluoromethyl-benzoylamino)-benzyl]-1H-pyrazol-4-yl}-acetic acid
[0240]
[0241] Intermediate 1.1.1
[0242] (1-Benzyl-3,5-dimethyl-1H-pyrazol-4-yl)-acetic acid methyl ester
[0243] (1-Benzyl-3,5-dimethyl-1H-pyrazol-4-yl)-acetic acid (1.00 g, 4.1 mmol) was dissolved in 3N methanolic HCl (7.5 mL) and stirred at room temperature for 18 hours . with NaHCO 3 The reaction mixture was neutralized with aqueous solution and extracted with dichloromethane. The organic layer was treated with MgSO 4 X was dried and concentrated under reduced pressure.
[0244] Yield: 963mg
[0245] ESI mass spectrum: [M+H] + =259
[0246] Retention time HPLC: 2.05 minutes (method A)
[0247] Intermediate 1.1.2 (via nitration)
[0248] [3,5-Dimethyl-1-(4-nitro-benzyl)-1H-pyrazol-4-yl]-acetic acid methyl ester
[0249] Under cooling, (1-benzyl-3,5-dimethyl-1H-pyrazol-4-yl)-acetic acid methyl ester (intermediate 1.1.1, 3.10 g, 12.0 mmol) was dissolved in concent...
Embodiment 11
[0266] {3,5-Dimethyl-1-[4-(4-trifluoromethyl-benzoylamino)-benzyl]-1H-pyrazol-4-yl}-acetic acid
[0267] Coupling: To [1-(4-amino-benzyl)-3,5-dimethyl-1H-pyrazol-4-yl]-acetic acid methyl ester (Intermediate 1.1.3, 85mg, 0.26mmol) To a solution in dimethylformamide (1 mL) was added 4-(trifluoromethyl)benzoic acid (62 mg, 0.32 mmol), diisopropylethylamine (90 μL, 0.53 mmol) and TBTU (94 mg, 0.29 mmol). The reaction mixture was stirred at room temperature for 18 hours. with K 2 CO 3 The reaction mixture was treated with aqueous solution (2M, 0.15 mL) and filtered through Alox B, eluting with 10% methanol in dichloromethane. Saponification: The volatiles were removed under reduced pressure and the remaining residue was treated with aqueous NaOH (4M, 0.2 mL). Via preparative reverse phase HPLC (gradient is methanol in water+0.1%NH 3 ) to purify the mixture.
[0268] Yield: 44mg
[0269] ESI mass spectrum: [M+H] + =432
[0270] Retention time HPLC: 1.94 minutes (method A) ...
Embodiment 21
[0280] {3,5-Diethyl-1-[4-(4-trifluoromethyl-benzoylamino)-benzyl]-1H-pyrazol-4-yl}-acetic acid
[0281]
[0282] Intermediate 2.1.1
[0283] [3,5-Diethyl-1-(4-nitro-benzyl)-1H-pyrazol-4-yl]-tert-butyl acetate
[0284] [3,5-Diethyl-1-(4-nitro-benzyl)-1H-pyrazol-4-yl]-acetic acid tert-butyl ester was prepared according to intermediate 1.1.2, after alkylation In the reaction, (3,5-diethyl-1H-pyrazol-4-yl)-tert-butyl acetate (prepared according to WO2007 / 141267) was used instead of (3,5-dimethyl-1H-pyrazole-4 -yl)-methyl acetate.
[0285] Intermediate 2.1.2
[0286] [1-(4-Amino-benzyl)-3,5-diethyl-1H-pyrazol-4-yl]-tert-butyl acetate
[0287] [1-(4-Amino-benzyl)-3,5-diethyl-1H-pyrazol-4-yl]-acetic acid tert-butyl ester was prepared according to intermediate 1.1.3 and used in the hydrogenation reaction Intermediate 2.1.1 replaced Intermediate 1.1.2.
[0288] ESI mass spectrum: [M+H] + =344
[0289] Retention time HPLC: 1.90 minutes (method A)
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com