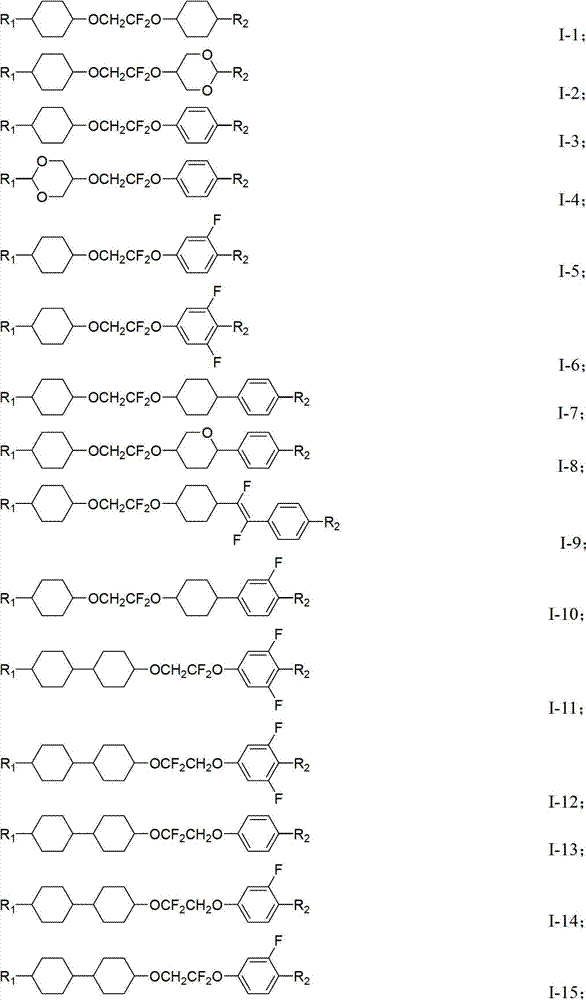
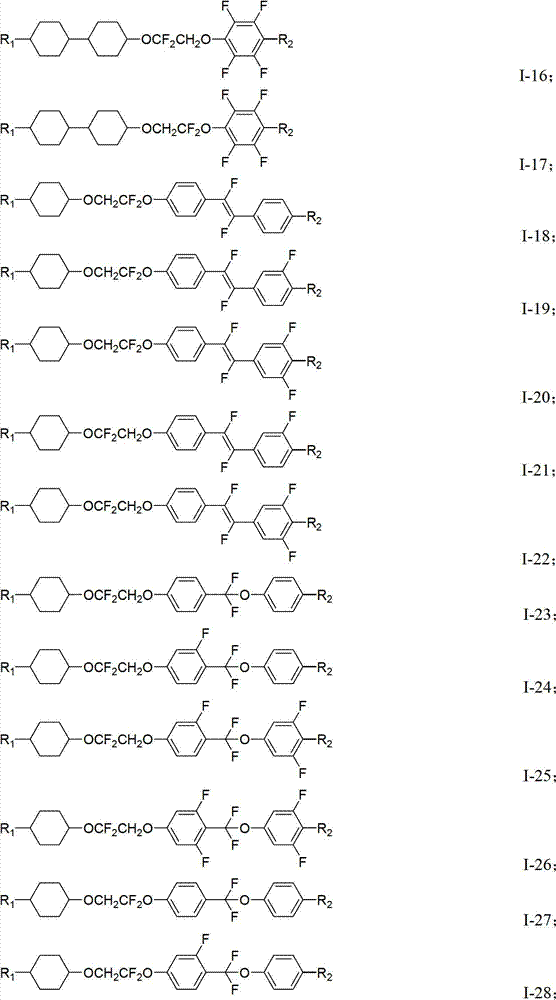
Novel difluoro ethylene diether liquid crystal and its composition
A compound and free technology, applied in the direction of liquid crystal materials, ether preparation, ester reaction to ether, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The synthetic route of the prepared compound HCLC-11H is shown in the following formula, and its specific process steps are as follows:
[0064]
[0065] 1) Synthesis of HCLC-11H-03
[0066] Add 6.54g procyclophenol and 180mL dimethylimide (DMF) to a 250mL three-necked flask, dissolve, add 2.04g NaH under nitrogen protection, slowly add 7.92g ethyl difluorobromoacetate, and raise the temperature to 60°C after the addition is complete React for 1 hour, post-treatment, acidify with dilute hydrochloric acid, add 180 mL of water, extract with 180 mL of ethyl acetate × 2, combine the organic phases and wash with 180 mL of water once, extract the water washing liquid once with 180 mL EA, combine all organic phases, wash with water for 3 times, and wash with brine After three times, it was dried and concentrated to obtain a crude product, which was then purified by petroleum ether column chromatography, concentrated and dried to obtain 9.7 g of HCLC-11H-03. The theoretical...
Embodiment 2
[0075] The synthetic route of the prepared compound HCLC-11J is shown in the following formula:
[0076]
[0077] The synthesis method of HCLC-11J is synthesized according to the synthesis steps of HCLC-11H, using trans-(1'-propyl-trans-4-cyclohexyl) cyclohexanol instead of propanol, and then through HCLC-11H-02 and The synthesis steps of HCLC-11H-01 are to obtain HCLC-11J-01; HCLC-11J-01 is combined with 2,3-difluoro-4-ethoxyphenol to obtain the compound HCLC-11J according to the synthesis method of HCLC-11H .
[0078] 1 H NMR (300MHz, CDCl 3 )δ6.79–6.54(m,2H),4.83(t,J=41.7Hz,2H),4.05(q,J=11.8Hz,2H),3.73–3.59(m,1H),1.93(dt,J =14.5,11.8Hz,2H),1.86–1.74(m,6H),1.50–0.99(m,18H),0.94–0.78(m,3H).
Embodiment 3
[0080] The synthetic route of the prepared compound HCLC-11M is shown in the following formula:
[0081]
[0082] Also referring to the synthesis method of HCLC-11H, 2,3-difluoro-4-ethoxyphenol was used instead of 3,4,5-trifluorophenol to react with HCLC-11J-01 to prepare compound HCLC-11M.
[0083] 1 H NMR (300MHz, CDCl 3 )δ7.50-7.21(m,2H),7.04-6.73(m,2H),6.77-6.57(m,2H),5.01(t,J=41.7Hz,2H),4.05(q,J=11.8Hz ,2H),2.53-2.28(m,1H),1.91(dt,J=15.0,11.6Hz,2H),1.79(dt,J=15.5,11.6Hz,2H),1.51-1.11(m,10H), 1.02(dt,J=15.7,11.7Hz,2H),0.93-0.82(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com