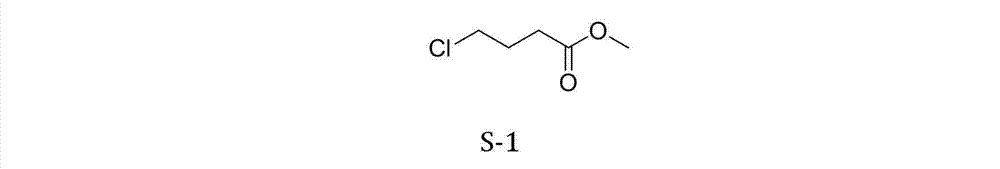
Synthetic method of methyl 4-chlorobutyrate
A technology of methyl chlorobutyrate and a synthesis method, which is applied to the synthesis field of organic compounds, can solve the problems of difficult disposal of waste gas and harsh reaction conditions, and achieves the effects of less three wastes, stable yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, a kind of synthetic method of methyl 4-chlorobutyrate:
[0023] Into a 250mL reactor, add 43g (0.5mol) of γ-butyrolactone, 40mL (1.5mol) of methanol, and 1.36g (0.01mol) of zinc chloride. Start stirring, and add 34.3g (0.25mol) of phosphorus trichloride dropwise at normal pressure and 50°C for 30 minutes. After dropping, keep warm and continue to react for 1 hour. The reaction solution was distilled under reduced pressure, and the fraction at 80-85° C. was collected under a pressure of 25 mm Hg to obtain 65.1 g of methyl 4-chlorobutyrate. The mass content is 99.1%, and the yield is 94.5%.
Embodiment 2
[0024] Embodiment 2, a kind of synthetic method of methyl 4-chlorobutyrate:
[0025] Into a 250mL reactor, add 43g (0.5mol) of γ-butyrolactone, 60mL (1mol) of methanol, and 1.36g (0.01mol) of zinc chloride. Start stirring, and add 34.3 g (0.25 mol) of phosphorus trichloride dropwise at normal pressure at 50°C for 30 minutes. After dropping, keep warm and continue to react for 1 hour. The reaction solution was distilled under reduced pressure, and the fraction at 80-85°C was collected under a pressure of 25 mm Hg to obtain 58.4 g of methyl 4-chlorobutyrate with a content of 99.0% and a yield of 85.5%.
Embodiment 3
[0026] Embodiment 3, a kind of synthetic method of methyl 4-chlorobutyrate:
[0027] Into a 250mL reactor, add 43g (0.5mol) of γ-butyrolactone, 100mL (2.5mol) of methanol, and 1.36g (0.01mol) of zinc chloride. Start stirring, and add 34.3 g (0.25 mol) of phosphorus trichloride dropwise at normal pressure at 50°C for 30 minutes. After dropping, keep warm and continue to react for 1 hour. The reaction solution was distilled under reduced pressure, and the fraction at 80-85°C was collected under a pressure of 25 mm Hg to obtain 63.8 g of methyl 4-chlorobutyrate with a content of 99.1% and a yield of 93.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com