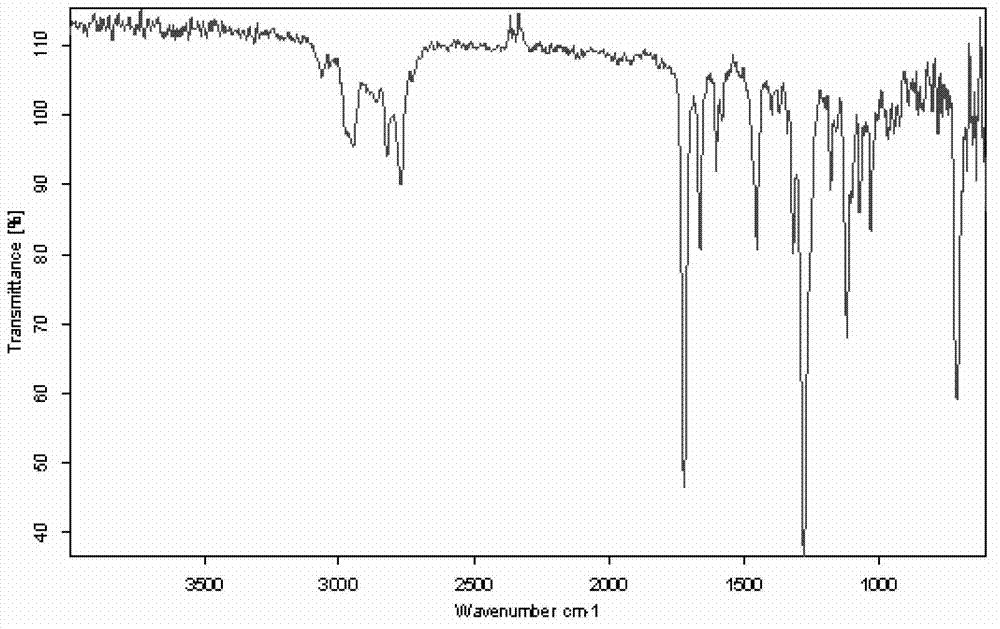
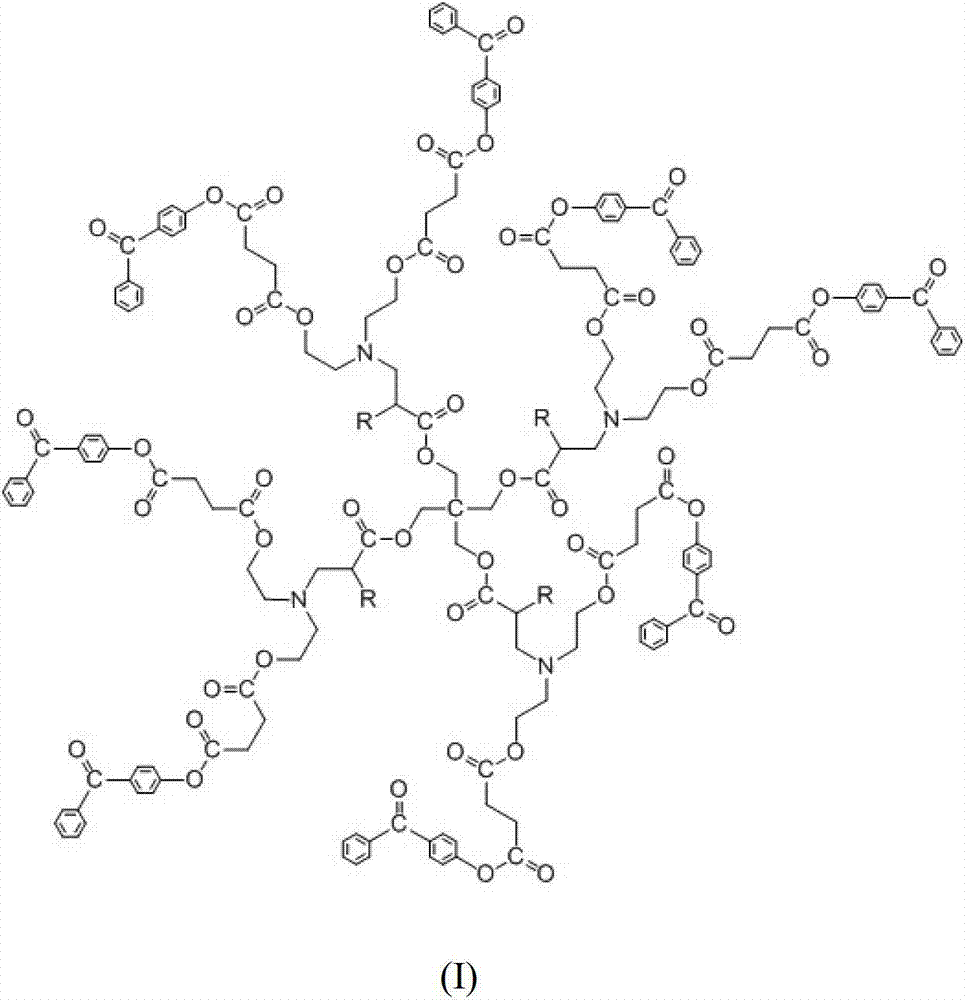
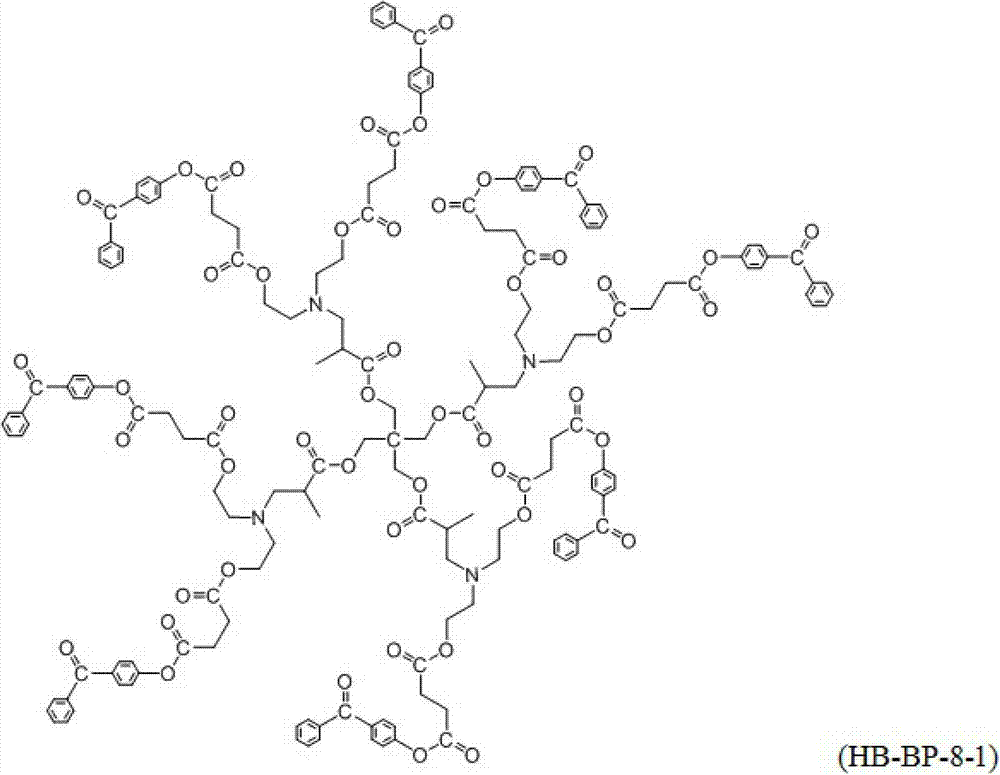
Highly branched macro-molecule photoinitiator and preparation method thereof
A photoinitiator and macromolecular technology, applied in the field of photoinitiator and its preparation, can solve the problems of precipitation of initiator, easy volatility of photoinitiator, restricted application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Highly branched macromolecular photoinitiator C 173 h 168 N 4 o 48 (named: HB-BP-8-1) preparation:
[0031] (1) Add diethanolamine (315.2g, 3.0mol) and 2-methyl methacrylate (300.4g, 3.0mol) into a 1000ml three-necked flask filled with 300ml of methanol, and stir at 30°C for 6 hours , then the solvent methanol was removed by rotary evaporation, extracted once with anhydrous ether, and then the ether was removed by rotary evaporation to obtain a light yellow liquid (533.4g), which was one of the monomer compounds IV containing three functional groups, namely: 2- Methyl-(N,N-dihydroxyethyl)-3-amino-benzoic acid methyl ester, the yield is 86.7%.
[0032] (2) Take the product 2-methyl-(N,N-dihydroxyethyl)-3-amino-methyl acrylate (255.0g, 1.2mol) in the above (1), pentaerythritol (40.8g , 0.3mol), they were added into a 500ml three-necked flask, and 1.0g of p-toluenesulfonic acid was added at the same time, heated to 140°C, stirred and reacted at this temperature for 5 ...
Embodiment 2
[0036] Highly branched macromolecular photoinitiator C 169 h 160 N 4 o 48 (named: HB-BP-8-2) preparation:
[0037] (1) Get diethanolamine (315.2g, 3.0mol) and methyl acrylate (258.4g, 3.0mol) respectively and join in the 1000ml three-neck flask that fills 300ml methanol, take the same processing method as Example 1, and finally obtain a The light yellow liquid (458.8g) is a monomer compound containing three functional groups: N,N-dihydroxyethyl-3-amino-methyl acrylate, and the yield is 80%.
[0038](2) Take the product N in the above (1), N-dihydroxyethyl-3-amino-methyl acrylate (229.4g, 1.2mol), pentaerythritol (40.8g, 0.3mol), and mix them Join in the there-necked flask of 500ml, add the p-toluenesulfonic acid of 1.0g simultaneously, carry out the same treatment process of step (2) among the embodiment 1, obtain the hyperbranched compound that end group contains 8 hydroxyls: four-( N,N-dihydroxyethyl)-propylcarboxy-isopentane (200.9 g), yield 86.7%.
[0039] (3) Take t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com