A tissue cryopreservation solution that can maintain cell viability
A technique for cryopreservation and tissue block, which is applied in the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] cryopreservation of adipose tissue
[0088] Experimental Materials
[0089] Adipose tissue: take 20ml of fresh sterile adipose tissue from the operating room
[0090] Freezing solution: KSR solution of 80vol%KSR+10vol%DMSO+10vol% trehalose, wherein the concentration of the KSR solution of trehalose is 2mol / l.
[0091] Experimental group
[0092] Panel A: cells were extracted from fresh adipose tissue and cultured
[0093] Group B: cells were extracted from frozen adipose tissue and cultured
[0094] experimental method
[0095] (1) Processing of fresh adipose tissue
[0096] Place the fresh adipose tissue in a sterile bottle under aseptic conditions, and wash with DPBS or saline for 3-4 times to remove the miscellaneous cells on the surface of the adipose tissue. Then use sterilized scissors to cut the tissue pieces (approximately 1mm 3 size) to facilitate cryopreservation of tissues.
[0097] The shredded tissues were equally divided into two groups, group A and ...
Embodiment 2
[0105] Recovery and inoculation of adipose tissue
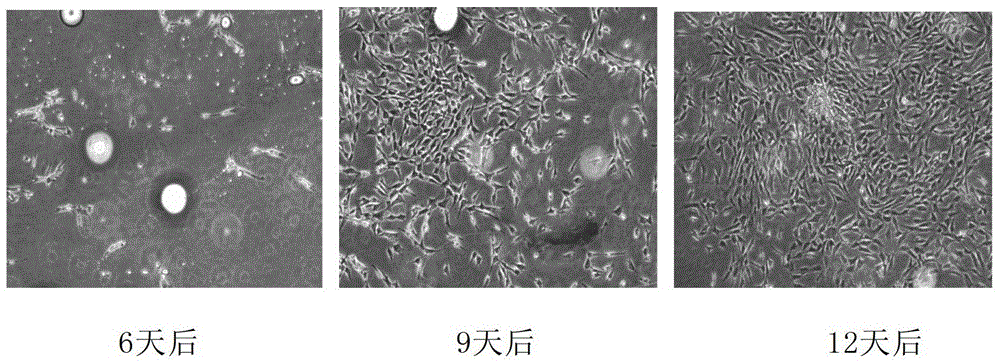
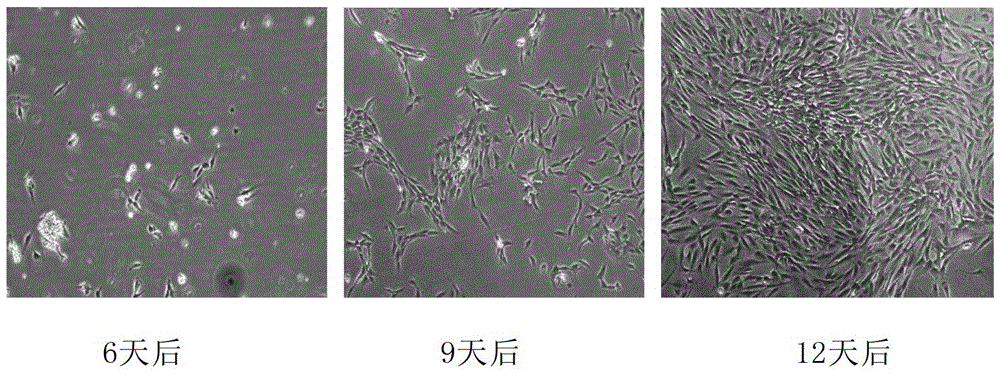
[0106] After 3 months, the cryopreservation tube containing the frozen adipose tissue was quickly placed in a 37°C water bath. After the tissue was dissolved, the tissue was taken out, and the residual cryopreservation solution was washed with DMEM (Sigma Company). Inoculate the resuscitated tissue into a culture flask of the same size and specification as Group A, add culture medium, and place at 37°C, CO 2 cultured in an incubator. Tissues were inoculated, and the tissue was completely changed after 3 days. The adherent growth of cells was observed under a microscope. Also make a record every 3 days and take pictures. The result is as figure 2 shown. After 3 days, no adherent cells were found; after 6 days, very few adherent cells were found, and the adherent cells were long spindle-shaped; after 9 days, the amount of adherent cells increased, and the cells grew well; after 12 days, there were many adherent cells, and...
Embodiment 3
[0113] Flow cytometry detection
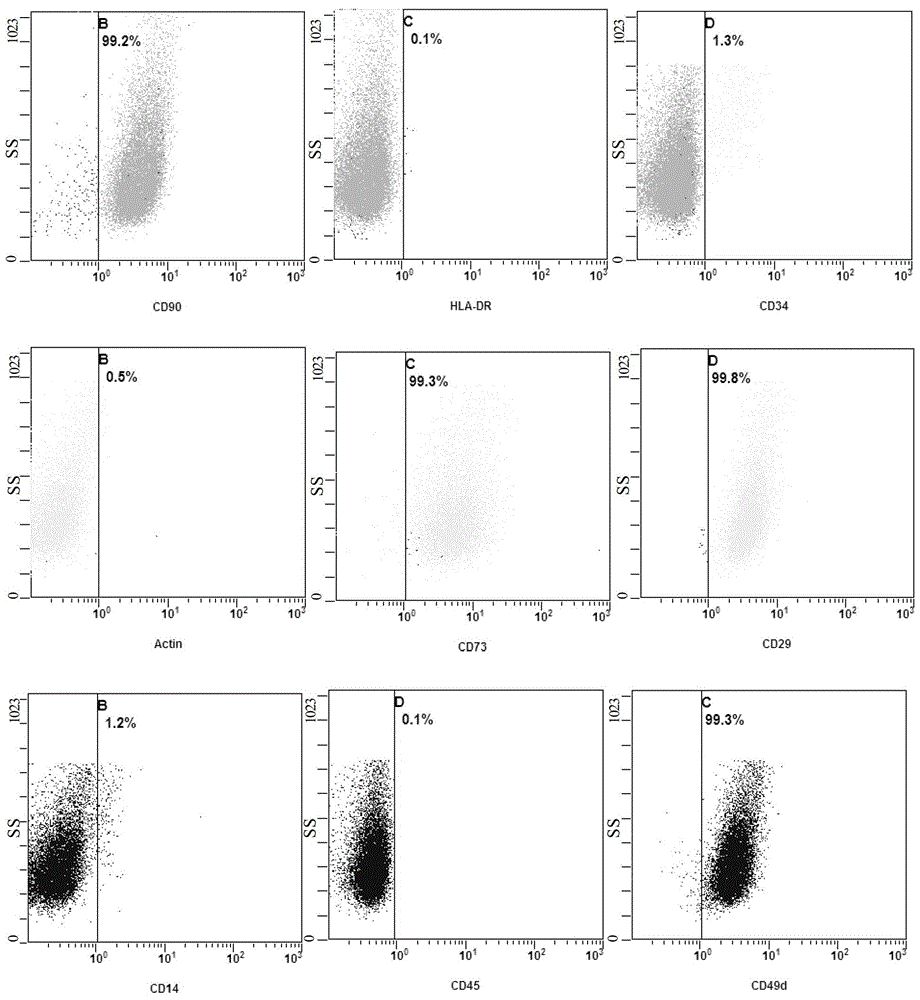
[0114] The above-mentioned cryopreserved P3 generation cells were taken, and after thawing, they were made into a cell suspension, and flow cytometric detection was performed to determine that the obtained cells were adipose-derived mesenchymal stem cells, and the cells were identified in terms of cell purity. Specifically, the cells were taken to make a cell suspension, and the density was adjusted to 1×10 5 mL -1 , Centrifuge at 800r / min (120g) for 5min, discard the supernatant, wash the resuspended cells with cold D-Hanks at 4°C, centrifuge the cell suspension again at 800r / min for 5min, and discard the supernatant. Then the cells were resuspended to 1 mL with D-Hanks, antibodies were added, and cell surface markers were detected by flow cytometry. The added antibodies were: human anti-CD29, CD73, CD49d, CD90, CD14, CD45, CD34, Actin and HLA-DR. The result is as image 3 and shown in Table 2. Among them, CD29, CD73, CD49d, and CD90 are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com