Chicken Cathelicidins antibacterial peptide and preparation method and application for same
A technology of pet30-cathl2s and antimicrobial peptides, which is applied in the field of chicken Cathelicidins antimicrobial peptides and its preparation, can solve the problems of high cost of antimicrobial peptides, inability to meet needs, and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Cloning and sequencing analysis of the complete coding gene of chicken Cathelicidin1, 2 antimicrobial peptides, including:
[0055] 1. Primer Design
[0056] According to the cathelicidins gene sequence (DQ092350, DQ092351) published in GenBank and the design principles of PCR primers, and with the help of computer software DNASTAR for auxiliary analysis, three pairs of PCR gene-specific primers were designed to amplify chicken cathelicidin1 , 2 The cDNA sequence of the coding gene, the primers are respectively CathL1 P1 / P2 and CathL2 P1 / P2 in the table, wherein P1 is the forward primer and P2 is the reverse primer. Primers were synthesized by Shanghai Boshang Company.
[0057] The primer sequences used in this example and Example 2 are shown in Table 1. In the CathL1S F / R, CathL2S F / R, primer pairs, the underlined bases represent the EcoR I and Xho I restriction endonuclease sites introduced in the upstream and downstream primers, respectively.
[0058] Table 1 Prim...
Embodiment 2
[0076] The genetic engineering preparation method of recombinant chicken Cathelicidins antimicrobial peptide fusion protein comprises the following steps:
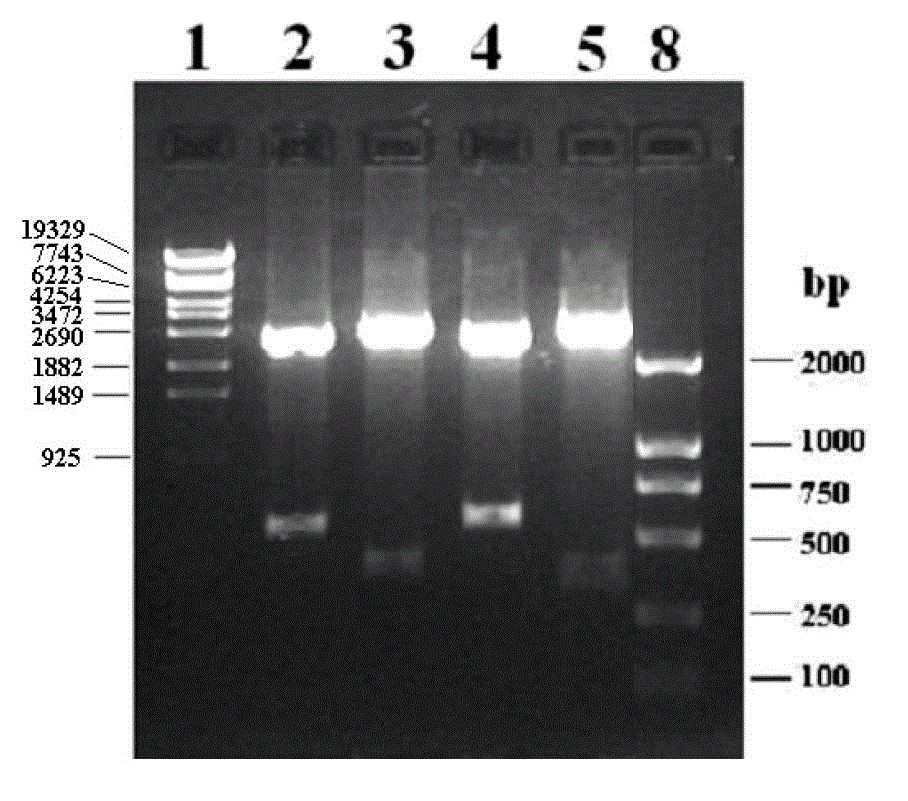
[0077] 1. Preparation of Chicken Cathelicidins 1, 2 Mature Peptide Coding Sequence and Construction of Fusion Expression Vector
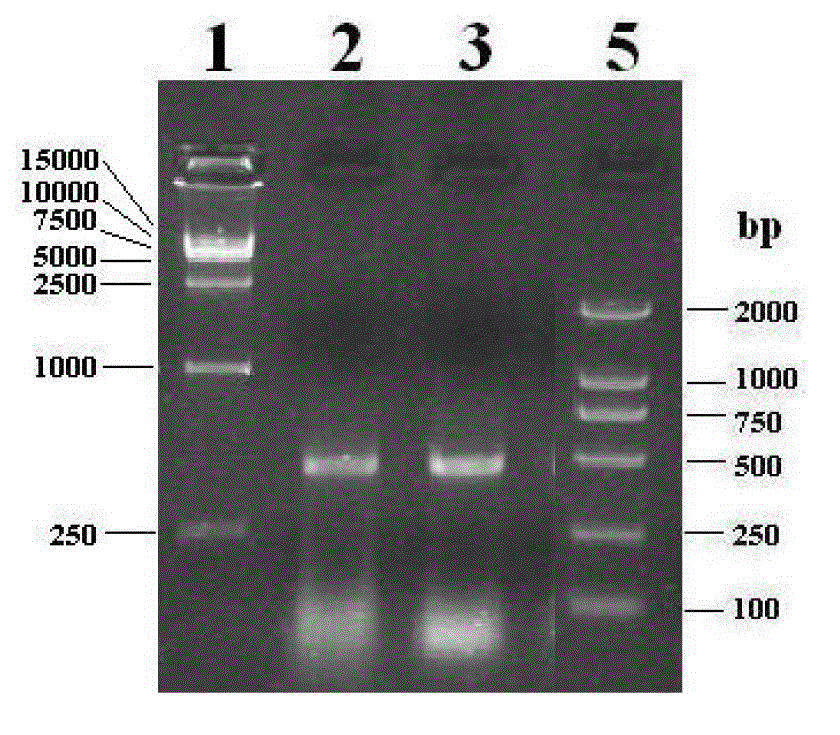
[0078] 1) Amplification of chicken Cathelicidins 1, 2 mature peptide coding sequence: According to the cloned chicken Cathelicidins 1, 2 antimicrobial peptide gene sequence and deduced amino acid sequence, design 2 pairs of specific primers CathL1S F / R, CathL2S F / R, the downstream primer has a stop codon TGA, and the primer sequence is shown in Table 1. Using the positive plasmids pMD-CathL1 and pMD-CathL2 containing the cDNA sequences of chicken Cathelicidins-1 and -2 genes as templates, the mature peptide coding sequences (~100bp) of chicken Cathelicidins 1 and 2 were amplified by PCR.
[0079] The reaction system is: 10×PCR Buffer (containing Mg 2+ ) 5.0 μL, dNTP (2.5 mmol / L) 4.0 μL, prim...
Embodiment 3
[0134] Optimization of Cathelicidins Fusion Protein Expression Conditions and Affinity Chromatography Purification of Expression Products
[0135] 1. Optimization of fusion protein expression conditions
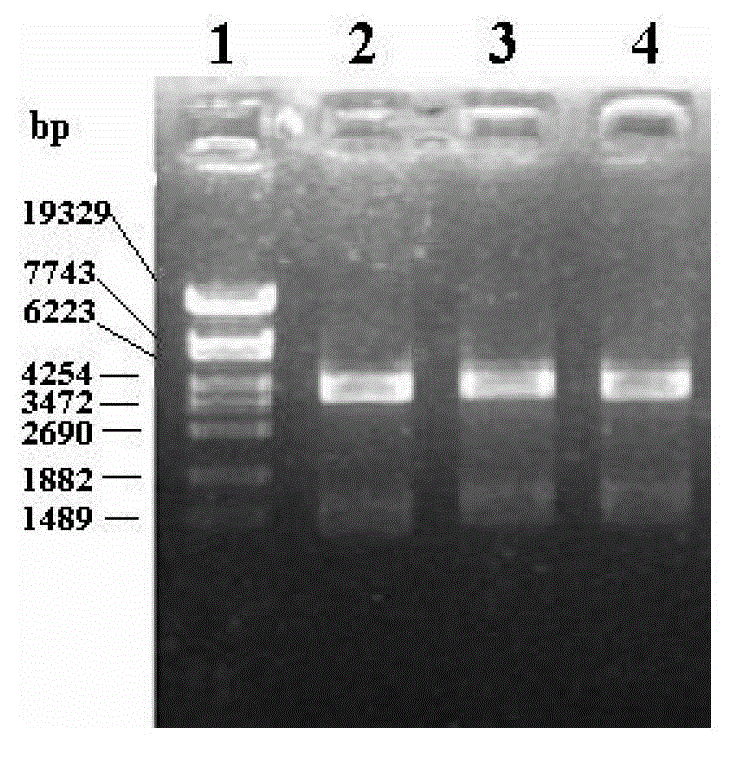
[0136] In order to increase the proportion of soluble components in the total protein expression, reduce the induction temperature (to 25°C or 15-20°C), prolong the induction time (3h-8h), and adjust the concentration of the inducer (the concentration of IPTG is 0.4mmol respectively) / L, 0.8mmol / L, 1.0mmol / L, 1.2mmol / L) and other methods to inhibit the formation of inclusion bodies, thereby simplifying the purification operation.
[0137] By using hypertonic medium (ie LB / SB medium), low temperature (25°C or 15~20°C) long-term induction, and adjusting the concentration of the inducer IPTG, etc., the formation of inclusion bodies can be inhibited and the soluble protein in the expression product can be increased. The purpose of the proportion. Experiments have shown that und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com