Yeast expressed chicken antibacterial peptide Cathelicidin 2 and preparation method and application thereof
An antibacterial peptide and expression vector technology, applied in the field of recombinant chicken Cathelicidin2 antibacterial peptides, can solve the problems of lack of processing and modification ability of gene expression regulation mechanism, difficulty in expressing antibacterial peptides, affecting the expression of antibacterial peptides, etc., achieving convenient artificial synthesis and easy amplification , the effect of simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The cloning of the mature peptide coding sequence of the silky chicken Cathelicidin2 antimicrobial peptide and the construction and identification of the secreted yeast expression vector specifically include the following steps:
[0040] 1. Design of primers
[0041] According to the Cathelicidin2 gene sequence of silky fowl published in GenBank (GenBank serial number: FJ938358) and the design principles of PCR primers, and with the aid of computer software DNASTAR for auxiliary analysis, a pair of PCR gene-specific primers were designed to amplify chickens. The mature peptide coding sequence of Cathelicidin2 gene, the primers are respectively pCathL2F / R in the table, where pCathL2F is the forward primer and pCathL2R is the reverse primer. At the same time, according to the known gene sequence structure, a pair of universal primers 5'AOX1 / 3'AOX1 of the eukaryotic expression vector pPICZαA were synthesized, and the primers were all synthesized by Invitrogen.
[0042] The prime...
Embodiment 2
[0051] The genetic engineering preparation method of recombinant chicken Cathelicidin2 antimicrobial peptide fusion protein includes the following steps:
[0052] 1. Construction of Pichia pastoris genetically engineered bacteria and selection of transformants
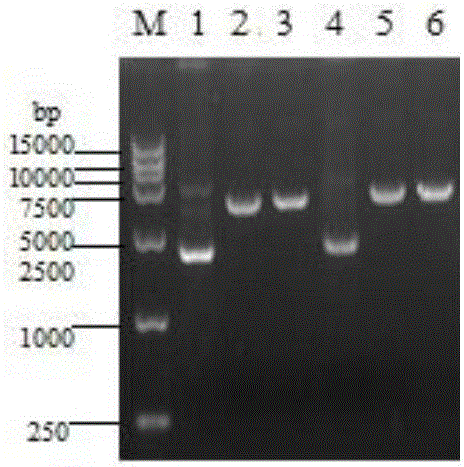
[0053] The recombinant expression plasmid pPICZαA-CathL2 and pPICZαA plasmid were subjected to single enzyme digestion treatment with endonuclease SacI, and the results are as follows figure 2 As shown, the linearized plasmid can be obtained. The linearized plasmids pPICZαA-CathL2 and pPICZαA are mixed with the prepared yeast X-33 competent cells in a quartz cup, and then placed in the electrotransmitter tank, and the parameters are adjusted to 1.5kV. , 25μF, 200Ω, 0°C electric shock, the above plasmids were integrated into the PichiapastorisX-33 genome.
[0054] Take 80μL of the transformation solution and evenly spread it on a YPDS (containing 100μg / mL Zeocin) plate, culture at 28°C for 3-5 days until white colonies grow. ...
Embodiment 3
[0064] Example 3 Detection of antibacterial activity of recombinant chicken antibacterial peptide Cathelicidin2
[0065] The indicator strains used in the bacteriostasis test: Micrococcus luteus CMCC28001, Salmonella pullorum CVCC79201 (C79-1), Pasteurella multocida (CVCC474, C48-7) standard strains were purchased from the China Veterinary Drug Administration ; Pseudomonaspyocyanea and Escherichiacoli 1503 are clinically isolated and identified strains preserved in this laboratory. Specific steps are as follows:
[0066] 1. In vitro drug susceptibility test of standard strains such as Escherichia coli and Salmonella
[0067] According to the instructions of the drug susceptibility kit, 9 kinds of drug susceptibility test strips, including amoxicillin (10μg / tablet), amikacin (30μg / tablet), azithromycin (15μg / tablet), etc., are selected to protect against Escherichia coli, Salmonella, and more. 5 strains of Pasteurella sexta were tested for drug susceptibility as a control for the an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com