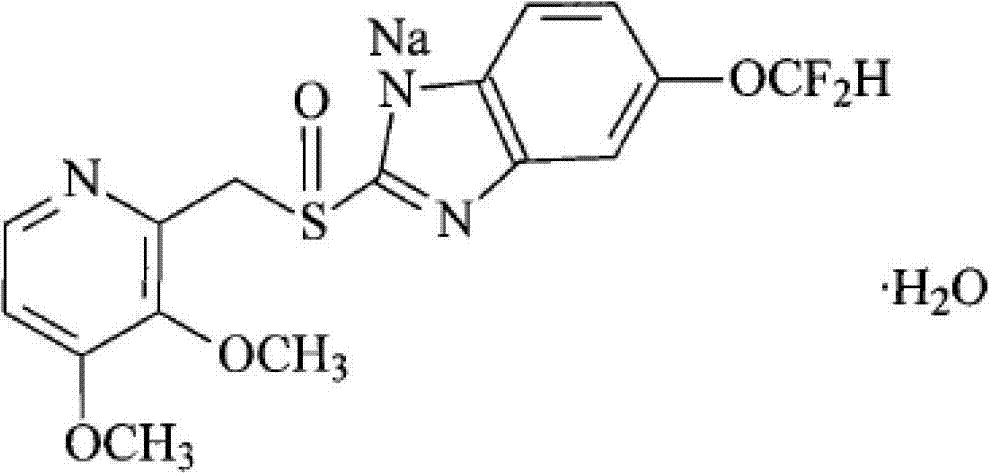
Pantoprazole sodium freeze-dried powder injection and preparation method thereof
A technology for pantoprazole sodium and freeze-dried powder injection, which is applied in the field of pharmaceutical preparations, can solve problems such as unfavorable large-scale production, difficult quality control, and complicated process steps, and achieves low content of related substances, simple preparation process and high stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0027] The preparation of embodiment 1-6 pantoprazole sodium freeze-dried powder
[0028] The prescription composition of table 1 pantoprazole sodium freeze-dried powder
[0029] components
Example 1
Example 2
Example 3
Example 4
Example 5
Example 6
40g
40g
40g
40g
40g
40g
50g
55
60
65
70
80
0.03g
0.02g
0.03g
0.04g
0.04g
0.05g
3g
6g
5g
4g
4g
3g
Appropriate amount
Appropriate amount
Appropriate amount
Appropriate amount
Appropriate amount
Appropriate amount
[0030] Preparation Process:
[0031] (1) Weigh pantoprazole sodium, lecithin and α-tocopherol, stir and dissolve in 500mL absolute ethanol, evaporate the ethanol under reduced pressure to obtain pantoprazole...
Embodiment 7
[0034] The stability investigation test of embodiment 7 pantoprazole sodium freeze-dried powder
[0035] Get respectively the pantoprazole sodium freeze-dried powder finished product prepared by the embodiment of the present invention 1-6, place high temperature 60 ℃ ± 2 ℃, place 10 days under the condition of light 4500Lx, investigate its character, acidity and solution clarity, related substance , content changes, test results are shown in Table 2.
[0036] Table 2 Test results of influencing factors (high temperature 60°C±2°C, light 4500Lx)
[0037]
[0038] Get respectively the pantoprazole sodium freeze-dried powder finished product prepared by the embodiment of the present invention 1-6, place temperature 40 ℃, place 6 months under the condition of humidity 75%, carry out accelerated test investigation, record its proterties, acidity and solution clarity Changes in degree, related substances and content, the test results are shown in Table 3.
[0039] Table 3 Accele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com