Recovery method of noble metal catalyst
The technology of a precious metal catalyst and a recovery method, which is applied in the chemical field, can solve the problems of difficult operation, dangerous operation, strong corrosiveness of aqua regia, etc., and achieves the effect of simple operation steps and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1) Use analytically pure methanol, 36% hydrochloric acid, and water as preparation materials, prepare a mixed solution according to the ratio of methanol: hydrochloric acid = 4:1, and mix well.
[0037] 2) Put the mixed solution in a glass pressure-resistant test tube, tightly cap the bottle, and heat in an oil bath at 150°C for three hours. Cool to room temperature and place in the refrigerator.
[0038] 3) Put the test tube in 2) into liquid nitrogen to freeze, add platinum nanoparticles, cap the bottle tightly, return the temperature to room temperature, put it in an oil bath and heat it to 150°C for three hours to obtain platinum precious metal nanoparticles dissolved on the surface. particle.
[0039] 4) Heating in an oil bath to 180° C. and heating for one hour to obtain platinum noble metal nanoparticles.
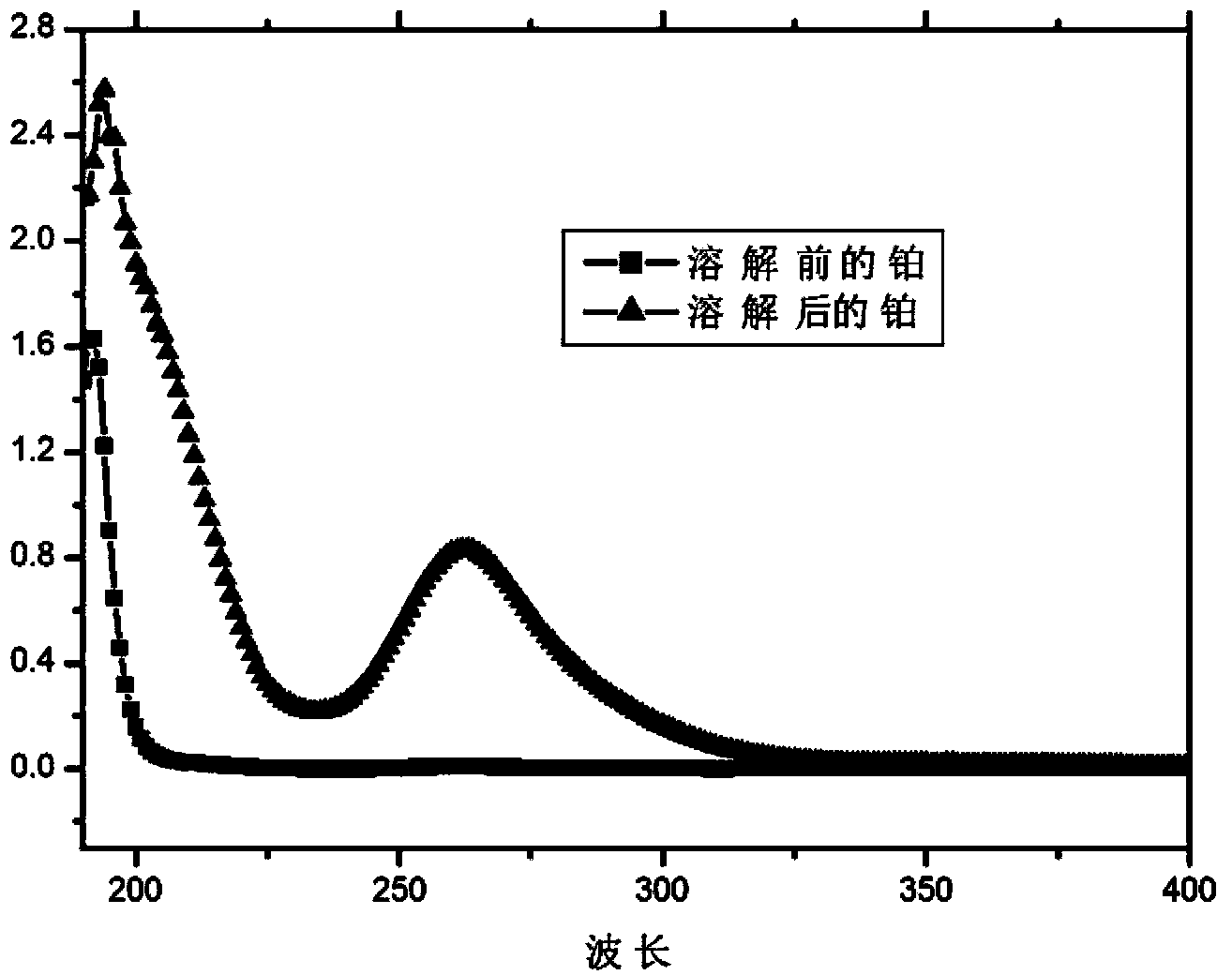
[0040] Such as figure 1 As shown, after the dissolution reaction, the unique absorption peak of platinum ions at 260 nm appeared in the solution, which ind...
Embodiment 2
[0042] 1) Use analytically pure methanol, 36% hydrochloric acid, and water as preparation materials, prepare a mixed solution according to the ratio of methanol: hydrochloric acid = 4:1, and mix well.
[0043] 2) Put the mixed solution in a glass pressure-resistant test tube, tightly cap the bottle, and heat in an oil bath at 150°C for three hours. Cool to room temperature and place in the refrigerator.
[0044] 3) Put the test tube in 2) into liquid nitrogen to freeze, add gold nanoparticles, cap the bottle tightly, return the temperature to room temperature, put it in an oil bath and heat it to 150°C for three hours to obtain gold nanoparticles dissolved on the surface .
[0045] 4) Heating in an oil bath to 180° C., and heating for one hour to obtain precious metal gold nanoparticles.
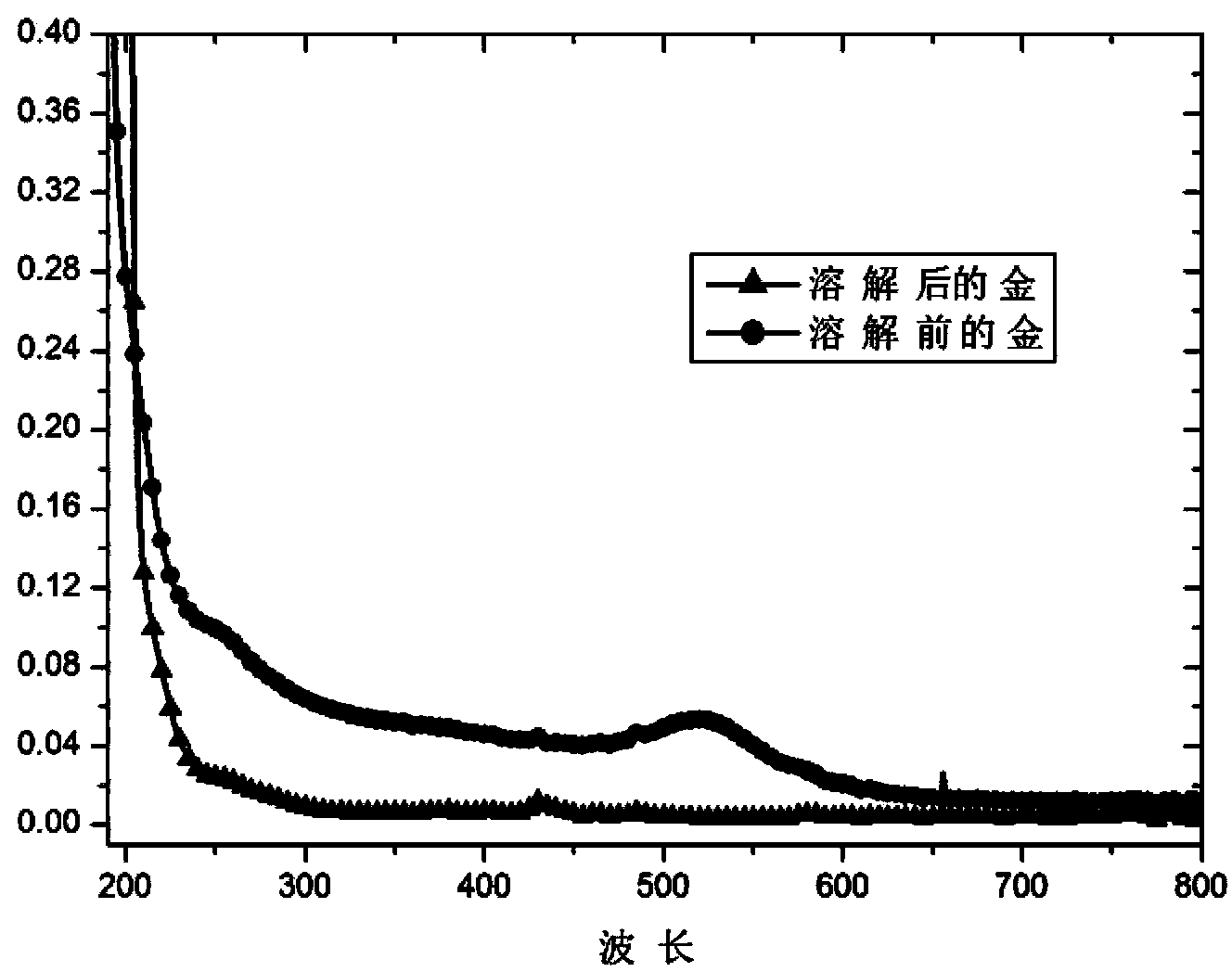
[0046] Such as figure 2 As shown, after the dissolution reaction, the ultraviolet absorption peak of the gold nanoparticles at 520 disappeared, indicating that the atomic state of gold h...
Embodiment 3
[0048] 1) Use analytically pure methanol, 36% hydrochloric acid, and water as preparation materials, prepare a mixed solution according to the ratio of methanol: hydrochloric acid = 2:1, and mix well.
[0049] 2) Put the mixed solution in a glass pressure-resistant test tube, tightly cap the bottle, and heat in an oil bath at 150°C for three hours. Cool to room temperature and place in the refrigerator.
[0050] 3) Put the test tube in 2) into liquid nitrogen to freeze, add platinum nanoparticles, cap the bottle tightly, return the temperature to room temperature, put it in an oil bath and heat it to 150°C for three hours to obtain platinum nanoparticles dissolved on the surface .
[0051] 4) Heating in an oil bath to 180° C. for one hour to obtain noble metal platinum nanoparticles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com